Written by: Conner Morton, MD (NUEM ‘26) Edited by: August Grace (NUEM ‘24)
Expert Commentary by: Dana Loke, MD, MS
Expert Commentary
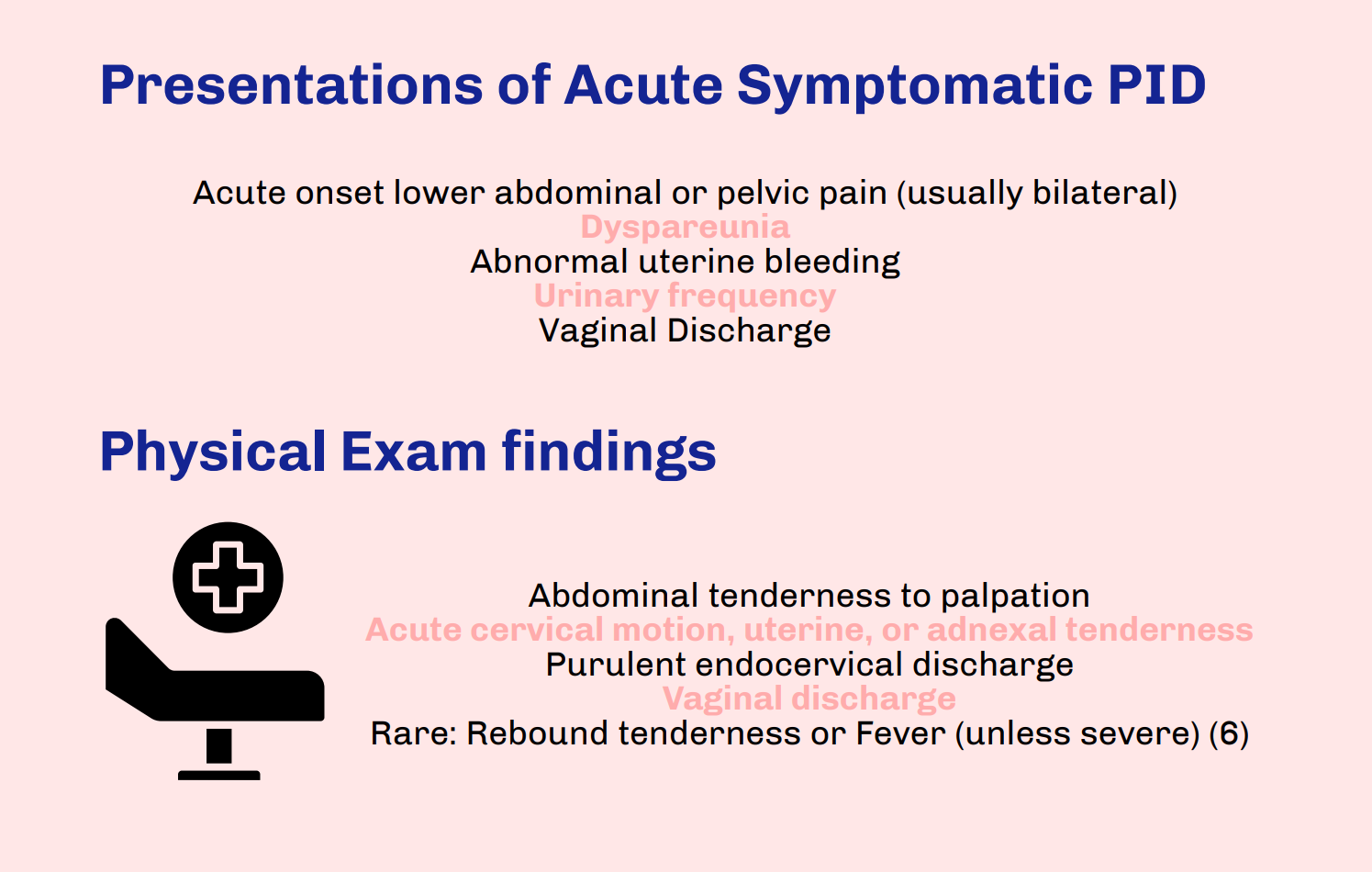
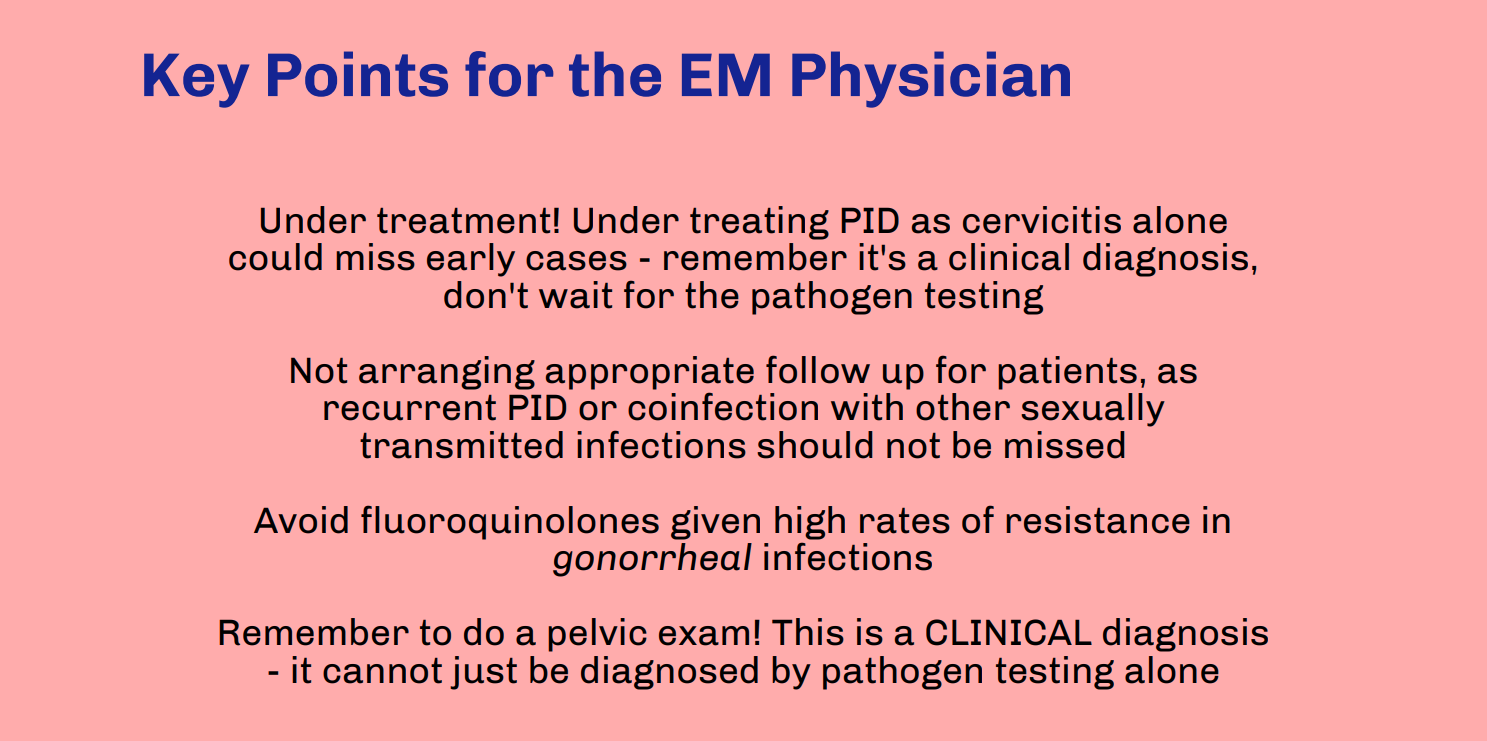
Thank you to Drs. Morton and Grace for this excellent infographic highlighting molar pregnancy and its relevance to Emergency Medicine providers. While rarer than other pregnancy issues seen in the Emergency Department, molar pregnancy is an important diagnosis for Emergency Medicine providers to be able to recognize, understand, and treat. As listed in the above post, symptoms of molar pregnancy can be similar to viable pregnancy and its complications, so it is important to obtain a timely ultrasound in any patient with unknown pregnancy location or if considering molar pregnancy. While molar pregnancy is not a difficult diagnosis to make as long as an ultrasound is done, it is important to be wary of its complications and treat appropriately. This includes monitoring vitals, specifically in consideration of hemorrhage and pre-eclampsia, with resuscitation and transfusion as needed. Consultation with OB/GYN should occur in the Emergency Department for immediate next steps, which will include procedural management. When updating the patient about the diagnosis and next steps, make sure to sensitively explain that molar pregnancy is nonviable. Lastly, make sure to complete a comprehensive review of systems and physical exam; molar pregnancy is associated with choriocarcinoma, which is known to spread widely and aggressively throughout the body.
Dana Loke, MD, MS
Assistant Professor
Emergency Medicine
University of Wisconsin-Madison
How To Cite This Post:
[Peer-Reviewed, Web Publication] Morton, C. Grace, A. (2024, Apr 1). Molar Pregnancy. [NUEM Blog. Expert Commentary by Loke, D. Retrieved from http://www.nuemblog.com/blog/molar-pregnancy