Written by: Courtney Premer-Barragan, MD, PhD (NUEM ‘25)
Edited by: Adam Payne, MD (NUEM ‘24)
Expert Commentary by: Kelsea Caruso, PharmD
Expert Commentary
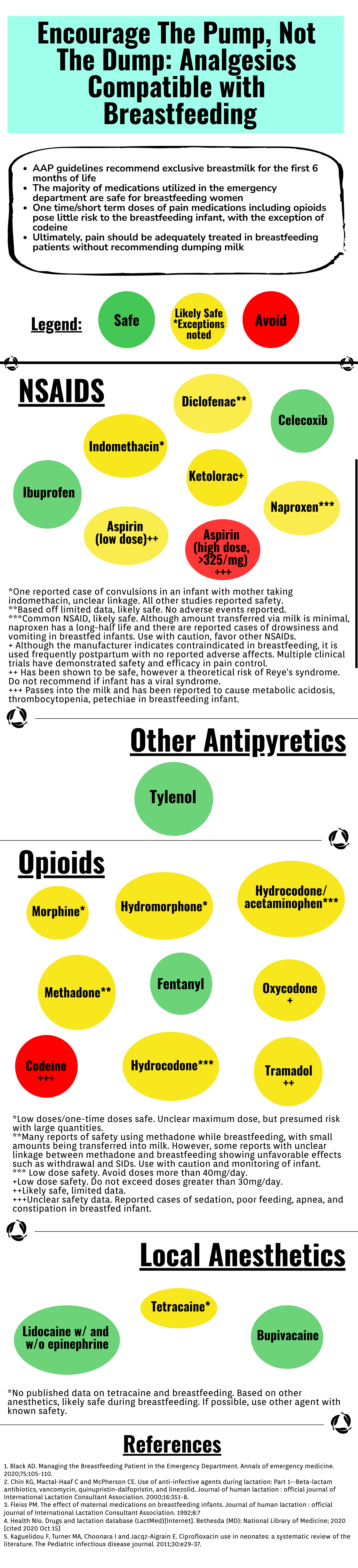
“Pump and dump” is definitely the easy way out for the emergency medicine provider, but this practice can have detrimental effects on the baby and on the mother. There is a false pretense that many medications are harmful to the breastfeeding infant, but this is not the case. The other consideration to have when thinking about medication use in breastfeeding is the medication effects on the mother’s lactation and the medication impact on breast milk production.
Ibuprofen by far has the most supporting evidence for use in breastfeeding women and this is a reasonable first line agent for treating many types of pain. Ketorolac is used frequently immediately after delivery and limited amount of drug is excreted in colostrum, but more may be excreted as milk supply increases thus increasing the risk of bleeding in the infant. Aspirin is excreted into breastmilk, and long-term use of high doses may cause bleeding along with metabolic abnormalities in the infant. That said, long-term use of low dose aspirin is likely safe.
If opioids are required for pain control, fentanyl is a reasonable choice for immediate pain control. Combination hydrocodone and acetaminophen is also an option when oral pain medications need to be utilized. The jury is (sort of) out on if oxycodone is safe during breastfeeding, and the baby should be monitored closely if oxycodone is selected for pain management.
Local anesthetics are very poorly absorbed by the infant, but still remain diligent about checking the specific maximum recommended dose for adults. My favorite database to find information on medications in lactation is LactMed, a database funded by the NIH. It is always safest to check this database before prescribing a medication to a lactating patient.
Kelsea Caruso, PharmD
Clinical Pharmacist
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Premer-Barragan, C. Payne, A. (2023, Jul 31). Breastfeeding Pharmacy Analgesics. [NUEM Blog. Expert Commentary by Caruso, K]. Retrieved from http://www.nuemblog.com/blog/breastfeeding-pharm-analgesics