Written by: Gabrielle Bunney, MD (NUEM PGY-2) Edited by: Alex Ireland, (NUEM PGY-4) Expert commentary by: Chris Richards, MD, MS
Introduction
Wake up strokes have always been a clinical conundrum. Current practice guidelines from the American Stroke Association on the treatment of acute ischemic strokes specify a maximum of 4.5 hours from time of symptom onset to the delivery of alteplase therapy. [1] However, patients often awaken with these symptoms or are unable to give a clear history of symptom onset and thus are not eligible for alteplase therapy. Initial non-contrast computed tomography can identify whether or not an acute hemorrhage is present, but confirmatory imaging for ischemic stroke involves magnetic resonance imaging (MRI). Studies are now looking at the utility of early MRI in the diagnostic and therapeutic pathways of acute ischemic stroke. These studies are specifically looking at a positive signal on diffusion-weighted imaging (DWI) and a negative signal on FLAIR imaging to identify recent cerebral infarction. Multiple studies have found that there is adequate sensitivity and specificity of DWI-FLAIR mismatch to suggest stroke onset within 4.5 hours. [2-4] Armed with these new data, this paper’s goal was to determine whether patients with an unknown time of symptom onset, but with a mismatch on DWI and FLAIR MRI imaging, would benefit from thrombolysis with intravenous alteplase.
Study
Thomalla G, Simonsen CZ, et. al “MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset | NEJM.” New England Journal of Medicine, Oxford University Press, www.nejm.org/doi/full/10.1056/NEJMoa1804355. [5]
Study Design
This study was a multi-center, randomized, double blind, and placebo controlled clinical trial. It involved 70 experienced stroke research centers in eight European countries. There was a central image-reading committee that reviewed all images for patient enrollment to evaluate inclusion and exclusion, and to arbitrate disagreements.
Population
Patients between the ages of 18 and 80 were eligible for the study if they clinically had an acute stroke and were able to perform their activities of daily living prior to this event. The patient had to awaken with these symptoms, be unsure about the time of onset secondary to confusion or aphasia, or have a timeline of symptoms greater than 4.5 hours, without an upper limit. 1,362 patients underwent screening. 859 were excluded, leaving 503 that were randomized. 254 of those were given alteplase and 249 received placebo.
Intervention Protocol
Selected patients underwent DWI and FLAIR MRI imaging. Those who had a mismatch, defined as an abnormal signal on DWI, but no signal on FLAIR, were then randomized. Excluded were those with intracranial hemorrhage, lesions larger than one third of the middle cerebral artery territory, those who were to undergo thrombectomy, those with severe stroke, defined as greater than 25 on the National Institute of Health Stroke Scale (NIHSS), and those that had any other contraindication to alteplase aside from time from last known normal. Those that were randomized into the alteplase group were given 0.9mg/kg of alteplase with 10% administered as a bolus and the rest given as an infusion over 60 minutes. Assessments were then conducted between 22 and 36 hours after randomization, between 5 and 9 days, and finally at 90 days.
Outcome Measures
This study had two end point measurements: efficacy and safety. The primary efficacy outcome measurement was favorable clinical outcome defined as a score of 0 to 1 on the modified Rankin scale 90 days after randomization. Secondary efficacy outcome measurements ranged from depression scores to activities of daily living measurements.
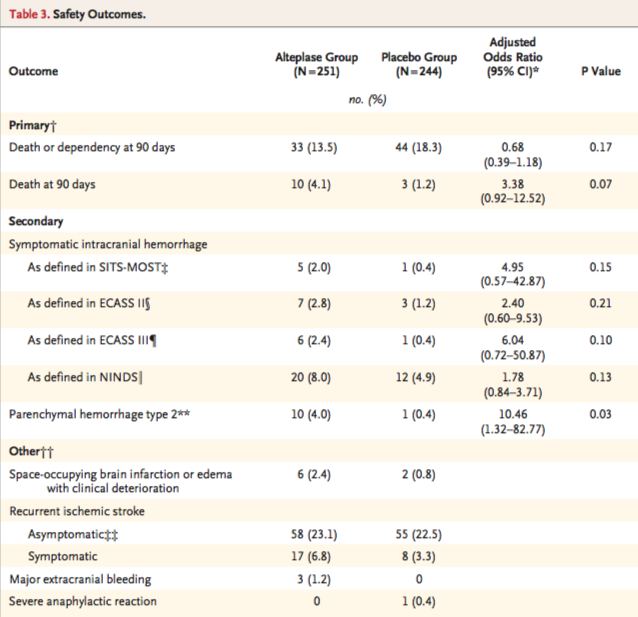
The primary safety outcome was death and a composite outcome of death or dependence (4-6 on the modified Rankin scale) at 90 days. Secondary safety endpoints were symptomatic intracranial hemorrhage and the incidence of parenchymal hematoma type 2 on MRI 22 to 36 hours after randomization.
Results
The demographics between the alteplase and placebo groups were similar for age, sex, and medical history. However, in the alteplase group there was a higher rate of intracranial occlusion of the internal carotid artery. The average time for treatment of the alteplase and placebo groups was 3.1 and 3.2 hours after symptom recognition, respectively.
Alteplase was found to be associated with favorable outcome at 90 days, with 53.3% in the alteplase group and only 41.8% in the placebo group having a modified Rankin score between 0 and 1 at 90 days, p=0.02. The secondary efficacy endpoints lacked power due to the fact that the study was terminated early because of a loss of funding. Table 2 from the original article describes the efficacy findings.
The safety groups were sized 251 in the alteplase group due to 5 patients not receiving alteplase and 244 in the placebo group due to 4 patients not receiving placebo. Death or dependency was found in 13.5% of the alteplase group and 18.3% of the placebo group, p=0.17. However, death at 90 days was higher in the alteplase group at 4.1%, while in the placebo group it was 1.2%, p=0.07. There were numerically more parenchymal hemorrhages in the alteplase group than in the placebo group, with 10 in the alteplase group and 1 in the placebo group. Table 3 from the original article describes the safety outcomes.
Interpretation
The primary efficacy outcome of this study, favorable functional outcome at 90 days, was higher in the group that received alteplase than in the group that received placebo and was statistically significant. Additionally, the primary safety outcome of death or disability was higher in the placebo group than in the alteplase group, though this was not found to be statistically significant. Death at 90 days was found to be numerically higher in the alteplase group than the placebo group, although not statistically significant. In extrapolating the data from the paper, the number needed to treat is 9.4 and the number needed to harm is 36.3. The ratio of these numbers suggests that treatment provides a greater benefit than risk.
There are several limitations to this study. The trial was stopped early due to lack of funding, and so we may be overestimating the benefit or underestimating the risk. The authors estimated that they needed approximately 800 patients to have sufficient power, yet enrolled only 503. Bleeding complications and death at 90 days were numerically higher in the alteplase group, though this was not statistically significant. Trials such as ATLANTIS A and B, and ASK, were all stopped early due to harm because of increased bleeding in the alteplase groups. [6-8] It is unknown whether the addition of 297 patients to meet the pre-specified enrollment target of 800 in the WAKE-UP trial would have resulted in statistical significance.
The population of this study had a median NIHSS of 6 out of 42, a relatively low stroke severity. The DAWN, DEFUSE, NINDS, and SITS-MOST trials, all significant studies in the progression of stroke research, had NIHSS medians of 17, 16, 14, and 12, respectively. [9-12] It is unclear if MRI-guided alteplase therapy would benefit patients with more severe strokes. Additionally, this paper excluded patients who were selected for thrombectomy. Patients selected for thrombectomy have large clot burdens in the internal carotid artery or middle cerebral artery that often have a modified Rankin score greater than 6. [13] By not including these patients, the WAKE-UP trial does not show the benefit of medical treatment in these sicker patients with a larger clot burden.
Lastly, the study was only performed in experienced research stroke centers with readily available diagnostic pathways and MRI. Of the 1,362 patients imaged and screened, only 37% met intervention criteria. Many did not have DWI-FLAIR mismatch, and some did not have any DWI lesion, suggesting a transient ischemic attack or a stroke mimic. A smaller hospital is unlikely to have the experience or equipment to be able to screen these more difficult patients for the few that can actually proceed to intervention.
Future Areas of Research
A replication of this study with additional subjects and sufficient power to confirm the beneficial effect of alteplase in MRI-guided thrombolysis would be the next step. Inclusion of alteplase plus thrombectomy in appropriate patients presenting after 4.5 hours with mismatch on DWI-FLAIR is another possible study. For example, TWIST (ClinicalTrials.gov Identifier: NCT03181360) and TIMELESS (ClinicalTrials.gov Identifier: NCT03785678) are two large clinical trials that will hopefully give more information about expanding the time window for thrombolysis. [14,15]
Review
MRI DWI-FLAIR mismatch may be able to allow more patients to receive alteplase therapy after an acute ischemic stroke
Alteplase still shows benefit for treating stroke even with an unknown timeline when used in conjunction with MRI DWI-FLAIR mismatch
Similar to prior studies, alteplase is associated with numerically higher instances of intracranial hemorrhage
Further research needs to be done to increase the power of this study
Expert Commentary
Really nice summary of the recent WAKE-UP* trial, and you bring up important considerations about both the pros and the cons of this study. WAKE-UP addressed an important clinical question: is it safe and effective for patients who have no other disqualifying reasons aside from their last known normal time to receive thrombolysis, if imaging shows a small area of infarction but a large area of ischemia? As you mention, intervention patients in the study: a) received intravenous tissue plasminogen activator (IV tPA) when otherwise they would not have, b) had increased odds of having a favorable outcome compared to standard of care, c) though with numerically greater instances of hemorrhage.
WAKE-UP fits into a narrative with two other important recent trials, DAWN* and DEFUSE-3*that studied the outcomes of patients with last known normal (LKN) times greater than conventional LKN time cut-offs (4.5 hours for IV tPA and 6 hours for endovascular therapy) and have found efficacy of reperfusion in these extended windows for select patients. A third trial, EXTEND,* has been presented in abstract form and has demonstrated clinical improvement in select AIS patients receiving IV tPA up to 9 hours from LKN time (https://abstractsonline.com/pp8/#!/4715/presentation/13367). Importantly, these trials that expand the time window for reperfusion used imaging-based criteria for inclusion: WAKE-UP used MR, DAWN used a non-contrast CT scan compared to severity of clinical syndrome, DEFUSE-3 used CT perfusion along with a computer software program to identify the infarcted “core” and the ischemic penumbra, and EXTEND required a penumbral mismatch on CT perfusion or MRI.
From a pathophysiological perspective, this makes sense. If imaging can identify a large area of ischemia and small area of infarction, reperfusion should potentially result in the salvage of at least some of those reversibly damaged cells. It should also result in less pronounced hemorrhagic side effects because the area of known infarction is less – remember, not only neurons die in infarcted brain, so do blood vessel endothelium cells, a contributing factor in post-reperfusion hemorrhage. [16]
Looking into the future of acute stroke care, these clinical trials give promise for individualized acute stroke treatment. Rather than being beholden to a rigid time cut-off (that evidence is showing is not one-size-fits-all), we can look to imaging to inform acute treatment decision. We have learned from subgroup analysis from DEFUSE-3 that some patients slowly progress in their stroke pathophysiology, meaning that even beyond 24 hours, some patients have a favorable core to penumbra ratio. [17] Other patients quickly progress in their stroke pathophysiology and may match their ischemic core to their salvageable penumbra well before traditional time-cut offs. [18]
It is possible that image-based selection criteria could be integral to the screening of all candidates for acute reperfusion therapy in the future. As WAKE-UP, EXTEND, DAWN, and DEFUSE-3 have shown us, there are some patients that can be reasonably considered for treatment beyond traditional time cut-offs. The same imaging criteria that extended the window for patients in these studies may be the same criteria that, in the future, could identify patients within the traditional time window who are likely to not benefit from treatment and who may have an increased risk of hemorrhagic conversion. One can image a patient without evidence of a salvageable penumbra presenting at 3 hours, for example, for whom the risks of IV tPA may, in fact, outweigh the potential benefits.
Lastly, I would hazard readers from interpreting the results of WAKE-UP, EXTEND, DAWN, and DEFUSE-3 as providing comfort in delaying thrombolysis or endovascular therapy for patients in extended time windows who would otherwise have indications for reperfusion. For IV tPA, longer delay is associated with increased risk of symptomatic intracranial hemorrhage and more timely treatment is associated with better outcomes. In the 2015 endovascular therapy trials, [19-23] even for patients within 6 hours of LKN, more timely treatment was associated with better outcomes. Even if the imaging protocols used in WAKE-UP, EXTEND, DAWN, and DEFUSE-3 can identify “slow progressors” that can be treated outside current treatment windows, these patients’ stroke are still progressing as time goes by. [18] Systems that promote timely evaluation of patients with stroke systems should be expected to help patients in extended time window, as they do patients within traditional time windows. {24,25]
Studies like WAKE-UP that test traditional inclusion and exclusion criteria for reperfusion give promise for safer and more effective stroke treatment. We look forward to future clinical trials, like TIMELESS* and TWIST*, that hopefully will give further clarity on this clinical question.
WAKE-UP [5]: Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke Trial
DAWN [10]: Diffusion Weighted Imaging (DWI) or Computerized Tomography Perfusion (CTP) Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention Trial
DEFUSE-3 [9]: Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 Trial
EXTEND [26]: EXtending the time for Thrombolysis in Emergency Neurological Deficits Trial
TIMELESS: Tenecteplase in Stroke Patients Between 4 and 24 Hours Trial (https://clinicaltrials.gov/ct2/show/NCT03785678)
TWIST: Tenecteplase in Wake-up Ischaemic Stroke Trial (https://clinicaltrials.gov/ct2/show/NCT03181360)
Chris Richards, MD, MS
Assistant Professor
Department of Emergency Medicine
Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Bunney G, Ireland A. (2019, Sept 9). Rise and Shine: A Review of the WAKE-UP Trial. [NUEM Blog. Expert Commentary by Richards C]. Retrieved from http://www.nuemblog.com/blog/wake-up-trial.
Other Posts You May Enjoy
Citations
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46-e110.
Aoki, Junya, et al. “FLAIR Can Estimate the Onset Time in Acute Ischemic Stroke Patients.” Journal of the Neurological Sciences, vol. 293, no. 1-2, 2010, pp. 39–44., doi:10.1016/j.jns.2010.03.011
Petkova, Mina, et al. “MR Imaging Helps Predict Time from Symptom Onset in Patients with Acute Stroke: Implications for Patients with Unknown Onset Time.” Radiology, vol. 257, no. 3, 2010, pp. 782–792., doi:10.1148/radiol.10100461.
Thomalla, Götz, et al. “DWI-FLAIR Mismatch for the Identification of Patients with Acute Ischaemic Stroke within 4·5 h of Symptom Onset (PRE-FLAIR): a Multicentre Observational Study.” The Lancet Neurology, vol. 10, no. 11, 2011, pp. 978–986., doi:10.1016/s1474-4422(11)70192-2.
Thomalla G, Simonsen CZ, et. al “MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset | NEJM.” New England Journal of Medicine, Oxford University Press, www.nejm.org/doi/full/10.1056/NEJMoa1804355.
Albers, Gregory W., et al. “ATLANTIS Trial.” Stroke, vol. 33, no. 2, 2002, pp. 493–496., doi:10.1161/hs0202.102599.
Clark, Wayne M., et al. “Recombinant Tissue-Type Plasminogen Activator (Alteplase) for Ischemic Stroke 3 to 5 Hours After Symptom Onset.” Jama, vol. 282, no. 21, Jan. 1999, p. 2019., doi:10.1001/jama.282.21.2019.
Donnan, G. A. “Streptokinase for Acute Ischemic Stroke with Relationship to Time of Administration: Australian Streptokinase (ASK) Trial Study Group.” JAMA: The Journal of the American Medical Association, vol. 276, no. 12, 1996, pp. 961–966., doi:10.1001/jama.276.12.961.
Albers, Gregory W, et al. “Thrombectomy for Stroke with Selection by Perfusion Imaging.” New England Journal of Medicine, vol. 378, no. 19, 2018, pp. 1849–1850., doi:10.1056/nejmc1803856.
Nogueira, Raul G, et al. “Thrombectomy 6 to 24 Hours after Stroke.” New England Journal of Medicine, vol. 378, no. 12, 2018, pp. 1161–1162., doi:10.1056/nejmc1801530.
“Tissue Plasminogen Activator for Acute Ischemic Stroke.” New England Journal of Medicine, vol. 333, no. 24, 1995, pp. 1581–1588., doi:10.1056/nejm199512143332401.
Wahlgren, Nils, et al. “Thrombolysis with Alteplase for Acute Ischaemic Stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an Observational Study.” The Lancet, vol. 369, no. 9558, 2007, pp. 275–282., doi:10.1016/s0140-6736(07)60149-4.
Campbell, Bruce C V, et al. “Endovascular Thrombectomy for Stroke: Current Best Practice and Future Goals.” Bmj, vol. 1, no. 1, 2016, pp. 16–22., doi:10.1136/svn-2015-000004.
“Tenecteplase in Wake-up Ischaemic Stroke Trial (TWIST).” ClinicalTrials.gov, clinicaltrials.gov/ct2/show/NCT03181360.
“Tenecteplase in Stroke Patients Between 4 and 24 Hours (TIMELESS).” ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03785678
Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA neurology 2014;71:1181-5. PMCID: 4592535.
Christensen S, Mlynash M, Kemp S, et al. Persistent Target Mismatch Profile >24 Hours After Stroke Onset in DEFUSE 3. Stroke 2019;50:754-7. PMCID.
Rocha M, Jovin TG. Fast Versus Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke: Clinical and Research Implications. Stroke 2017;48:2621-7. PMCID.
Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20. PMCID.
Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18. PMCID.
Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA neurology 2015:1-7. PMCID.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. PMCID.
Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. PMCID.
Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA 2015;313:1451-62. PMCID.
Higashida R, Alberts MJ, Alexander DN, et al. Interactions within stroke systems of care: a policy statement from the American Heart Association/American Stroke Association. Stroke 2013;44:2961-84. PMCID.
Churilov L, Ma H, Campbell BC, Davis SM, Donnan GA. Statistical Analysis Plan for EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial. International journal of stroke : official journal of the International Stroke Society 2018:1747493018816101. PMCID.