Written by: Em Wessling, MD (PGY-2) Edited by: Will Ford, MD (NUEM ‘19) Expert commentary by: Justin Morgenstern, MD
While the Joint Commission historically focused their urinary interests on CAUTI protection, there is room for improvement in how we care for those with community acquired urinary tract infections. In many emergency departments, patients with suspected pyelonephritis are having blood cultures drawn to screen for bacteremia. However, these cultures may be unnecessary and costly for most patients.
The Choosing Wisely campaign from the ABIM Foundation started in 2012 with the goal to reduce “the overuse [of medical testing] that does not add value for patients” (1). In 2015, Choosing Wisely, in collaboration with the Society for Healthcare Epidemiology of America, recommended that blood cultures should not be performed unless there are appropriate symptoms due to false positives leading to over treatment (2). When looking at emergency department data, the lack of utility of blood cultures in general holds true. A study from Glasgow found that only 1.4% of all blood cultures drawn in the emergency department were true positives. Of these, less than 15% (or less than 0.2% of all cultures drawn) were used to guide clinical treatment, regardless of the suspected source of infection (3).
Similarly, Choosing Wisely Australia recommends avoiding blood cultures if patients are not systemically septic and have a “direct specimen for culture,” including urine (4). However, researchers in Australia continue to debate if this Choosing Wisely recommendation is based on enough evidence to apply broadly, or if blood cultures would still be useful for specific, more complicated populations.
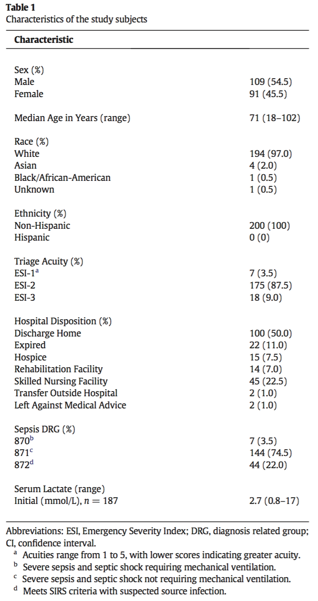
In patients with pyelonephritis, we can see that blood cultures rarely add clinical value. In 2017, it was shown that less than 10% of patients who were hospitalized for community acquired acute pyelonephritis had positive blood cultures (5). In the same study, only 2.3% of the cases had differing blood cultures when compared to urine cultures that resulted in a change of care (5). This was also demonstrated in a review article from 2005 that looked at the utility of blood cultures in immunocompetent, non-pregnant, adult patients and concluded that there was no use for blood cultures in this population (6). Blood cultures also have limited utility in predicting prognosis in patients with pyelonephritis. A recent study from Spain looked at all-cause mortality in pyelonephritis and urinary sepsis patients with bacteremia vs those without and found that here was no change (7). The same prospective study found no significant difference in length of stay and ICU transfers (7).
Blood cultures do occasionally have a role to play in the treatment of pyelonephritis. While the average person with an uncomplicated UTI or pyelonephritis may not have an indication for blood cultures, there are select populations for whom blood cultures show a distinct benefit. Initially, it was postulated that those groups would include those with instrumentation of the Genito-urinary tract and those who are immunocompromised (6). Recent studies suggest that blood cultures may also be helpful in patients recently treated with antibiotics, as they are at a higher risk for sterile urine culture but may still have a positive blood culture. Additionally, chronically ill patients may have polymicrobial urine cultures, for whom a single clinically relevant organism may be able to be isolated from a blood culture (8).
While there is a plethora of research to demonstrate that in pyelonephritis for which a urine cultures is available, blood cultures are often not clinically significant, researchers are still trying to parse out which select groups would benefit from them.
Expert Commentary
This is an excellent post that clearly comes to the right conclusion: blood cultures are not necessary for most patients with pyelonephritis. (In fact, I think it’s likely that even urine cultures are overused.)
Whenever we order a test, we should consider: how will the results change my management?
Blood cultures are occasionally used diagnostically (for endocarditis), but pyelonephritis is a clinical diagnosis. The results of the blood culture is not going to change our final diagnosis. Therefore, the only management change we could possible make based on the blood cultures is a change in antibiotics.
Our initial antibiotics cannot be guided by cultures, but luckily our empiric antibiotics are incredibly effective. There are only a handful of bacterial species that routinely cause urinary tract infections, and we have a handful of commonly used antibiotics, so we choose correctly most of the time. Even when the chosen antibiotic is reported as resistant on the culture, you will frequently find that the patient is better clinically. (In vitro antibiotic resistance is not the same as in vivo resistance.)
Only a small number of patients will have a positive blood cultures. Only a smaller number will have a positive culture demonstrating resistance to the original antibiotic. And an even smaller number will still be sick at the time that the culture is reported. For this small minority of patients, the culture will guide our new antibiotic choice, but considering the limited menu of antibiotics we use for UTIs, we probably could have made the same decision empirically, and we would be right most of the time. (Even in the era of multidrug resistance and ESBL, you should have a general sense of what antibiotics work in your community.)
However, that entire line of logic is unnecessary if you already took a urine culture. (The same line of reasoning can demonstrate why urine cultures are probably also overused, but I will admit that although I never send cultures in simple UTIs, I still send them in pyelonephritis.) Considering that it is the actual source of the infection, the urine culture is far more likely to grow the causative organism. So if you already have a test that will guide your antibiotic change in the case of resistance, the blood culture is completely redundant. It cannot help. So we should stop sending them.
Justin Morgenstern, MD
Emergency Medicine
Toronto, Canada
References
Levinson, Wendy, et al. "‘Choosing Wisely’: a growing international campaign." BMJ Qual Saf 24.2 (2015): 167-174.
Society for Healthcare Epidemiology of America. “Don’t Perform Urinalysis, Urine Culture, Blood Culture or C. Difficile Testing Unless Patients Have Signs or Symptoms of Infection. Tests Can Be Falsely Positive Leading to over Diagnosis and Overtreatment.” Choosing Wisely - An Initiative of the ABIM Foundation, ABIM Foundation, 1 Oct. 2015, www.choosingwisely.org/clinician-lists/shea-urinalysis-urine-culture-blood-culture-or-c-difficile-testing/.
Howie, Neil, Jan F. Gerstenmaier, and Philip T. Munro. "Do peripheral blood cultures taken in the emergency department influence clinical management?." Emergency Medicine Journal 24.3 (2007): 213-214.
Denny, Kerina J., and Gerben Keijzers. "Culturing conversation: How clinical audits can improve our ability to choose wisely." Emergency Medicine Australasia 30.4 (2018): 448-449.
Kim Y, Seo MR, Kim SJ, Kim J, Wie SH, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Pai H. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017;49:22-30.
Mills, Angela M., and Suzanna Barros. "Are blood cultures necessary in adults with pyelonephritis?." Annals of emergency medicine 46.3 (2005): 285-287.
Artero, Arturo, et al. "The clinical impact of bacteremia on outcomes in elderly patients with pyelonephritis or urinary sepsis: A prospective multicenter study." PloS one 13.1 (2018): e0191066.
Karakonstantis, Stamatis, and Dimitra Kalemaki. "Blood culture useful only in selected patients with urinary tract infections–a literature review." Infectious Diseases 50.8 (2018): 584-592.
How to Cite This Post
[Peer-Reviewed, Web Publication] Wessling, E, Ford, W. (2020, Mar 26). Blood Cultures in Suspected Simple Cystitis vs Pyelonephritis. [NUEM Blog. Expert Commentary by Morgenstern, J]. Retrieved from https://www.nuemblog.com/blog/bcx-cystitis