An engaging and discussion of the severe end of the spectrum of DVT
Hypertensive Emergency
An overview of diagnosis and management of Hypertensive Emergency.
STEMI to OMI: Rethinking who will benefit from PCI
EKG presentations of Occlusion Myocardial Infarction
AIVR in the Emergency Department
An overview of AIVR and its management.
Symptomatic Bradycardia: Considering the Differential Diagnosis
Written by: Keara Kilbane (NUEM ‘25) Edited by: Eric Power (NUEM ‘24)
Expert Commentary by: Seth Trueger, MD, MPH, FACEP
THE HEART CONDUCTION SYSTEM
"Normal” adult heart rates range from 60-100 beats per minute (BPM), with bradycardia defined as a heart rate of less than 60 BPM. Electrical impulses for each heartbeat begin at the sinoatrial (SA) node, then propagate through the atrium to the atrioventricular (AV) node, continuing down the Bundle of His, and lastly to each bundle branch, causing the ventricles to contract. Bradycardia can be physiologic, such as in individuals who have high levels of cardiovascular training. However, pathologic and/or symptomatic bradycardia results from a disruption in this electrical circuit [1].
What is symptomatic bradycardia [1-2]?
Defined as the presence of bradycardia, resulting in debilitating symptoms with lack of alternate explanation.
The most common symptoms include:
Lightheadedness
Syncope
Chest pain
Exercise intolerance
Fatigue
**Important note: The heart rate at which patients experience symptoms may vary based on their ability to increase stroke volume.
CARDIAC OUTPUT = STROKE VOLUME X HEART RATE
Treatment Algorithm for Symptomatic Bradycardia [2-4]:
What are common causes of symptomatic bradycardia [2]?
Myocardial Infarction
Medication
Sinus node dysfunction
Infectious Disease
Hypothermia
Metabolic Abnormalities (hypothyroidism, hyperkalemia, ect.)
Elevated intracranial pressure (ICP)
Genetic Conditions
Myocardial Infarction
Bradyarrhythmias occur in up to 25% of patients with an acute myocardial infarction, especially those involving the right coronary artery (RCA) which supplies the SA node in up to 60% of patients.
Treatment for bradycardia secondary to myocardial infarction is standard care for an occlusive MI, including emergent cardiology consult and cath lab activation, and loading the patient with anti-platelet medications [5-7].
Common Medications and Mechanisms of Causing Bradyarrhythmias
Beta Blockers and Non-Dihydropyridine Calcium Channel Blockers (Diltiazem and Verapamil)
Inhibit the automaticity of the SA node
Antiarrhythmics (Amiodarone, Adenosine, Flecainide)
Inhibits the SA and AV node
Acetylcholinesterase inhibitors (Donepezil, Neostigmine, Pyrodostigmine, Physostigmine)
Activates the parasympathetic nervous system which leads to inhibition of the automaticity of the SA node
Clonidine
Stimulation of central alpha-2-receptors, reducing the norepinephrine
Antidepressants (Citalopram, Escitalopram, Fluoxetine)
Sodium and calcium channel inhibition
Digoxin
Increases vagal tone
Anesthetics (Bupivacaine, Propofol)
Reduces sympathetic activity
Treatment depends on the medication involved. If high suspicion or known overdose, involve and consult your local Poison Center [8].
Sinus Node Dysfunction (Sick Sinus Syndrome) [9-10]:
Sick sinus syndrome is most commonly due to aging of the sinus node and surrounding atrial myocytes. It is often associated with severe bradycardia (HR<50 bpm. It is also associated with sinus pauses, arrests, and SA node block, and or a junctional escape rhythm. Treatment includes permanent pacemaker placement [9].
Hypothermia
Moderate to severe hypothermia can cause significant bradycardia leading to hypotension. Treatment includes removing all wet clothing, externally rewarming with bair huggers and warm blankets, administering warm IV fluids, active core rewarming including bladder and thoracic irrigation with warmed fluids). It is also important to remember that the differential to hypothermia itself is broad itself and not just limited to environmental exposure. For example, hypothyroidism, adrenal insufficiency, sepsis, neuromuscular disease, malnutrition, thiamine deficiency, hypoglycemia can all lead to hypothermia [11-13].
Decompensated Hypothyroidism (Myxedema Coma)
Classic symptoms of myxedema coma include:
Decreased mentation or delirium
Hypothermia
Bradycardia
Hyponatremia
Hypoglycemia
Hypoventilation
Hypotension
Common triggering events:
Infection
Medication non-adherence
Surgery or trauma
Myocardial infarction
CHF exacerbation
Cerebral Vascular Accident
GI bleed
Treatment includes IV atropine if unstable while treating the underlying condition (IV steroids, IV levothyroxine) [14].
Increased Intracranial Pressure
Classic triad of bradycardia, respiratory depression, and hypertension (Cushing reflex), concerning for brainstem compression and/or herniation [15].
Treatment includes treating the underlying condition, and stabilization through maneuvers including [15]:
Hyperventilation
Head of the bed elevation to maximize venous outflow
Ensure neck braces (c-collar) is appropriately placed (not too tight)
Hypertonic solutions like mannitol or hypertonic saline
Emergent craniotomy
Other Etiologies of Symptomatic Bradycardia [15-16]
Prolonged hypoxia
Severe electrolyte derangements (hyperkalemia)
Vagal response
Severe obstructive sleep apnea
Genetic channelopathies
References:
Spodick, D. H., Raju, P., Bishop, R. L., & Rifkin, R. D. (1992). Operational definition of normal sinus heart rate. The American Journal of Cardiology, 69(14), 1245–1246. https://doi.org/10.1016/0002-9149(92)90947-w
UpToDate. (n.d.). Www.uptodate.com. https://www.uptodate.com/contents/sinus-bradycardia?search=Bradycardia&source=search_result&selectedTitle=1~150&usage_type=default&disp
Spodick, D. H. (1992). Normal sinus heart rate: Sinus tachycardia and sinus bradycardia redefined. American Heart Journal, 124(4), 1119–1121. https://doi.org/10.1016/0002-8703(92)91012-p
ACLS Bradycardia Algorithm. (n.d.). ACLS Medical Training. https://www.aclsmedicaltraining.com/adult-bradycardia-algorithm/
A. A. J. Adgey, Geddes, J. S., Mulholland, H., Keegan, D., & Pantridge, J. F. (1968). INCIDENCE, SIGNIFICANCE, AND MANAGEMENT OF EARLY BRADYARRHYTHMIA COMPLICATING ACUTE MYOCARDIAL INFARCTION. The Lancet, 292(7578), 1097–1101. https://doi.org/10.1016/s0140-6736(68)91577-8
UpToDate. (n.d.). Www.uptodate.com. Retrieved June 11, 2024, from https://www.uptodate.com/contents/sinus-node-dysfunction-clinical-manifestations-diagnosis-and-evaluation?search=bradycardia&source=
ROTMAN, M., WAGNER, G. S., & WALLACE, A. G. (1972). Bradyarrhythmias in Acute Myocardial Infarction. Circulation, 45(3), 703–722. https://doi.org/10.1161/01.cir.45.3.703
Tisdale, J. E., Chung, M. K., Campbell, K. B., Hammadah, M., Joglar, J. A., Leclerc, J., & Rajagopalan, B. (2020). Drug-Induced arrhythmias: A scientific statement from the american heart association. Circulation, 142(15). https://doi.org/10.1161/cir.0000000000000905
Kusumoto, F. M., Schoenfeld, M. H., Barrett, C., Edgerton, J. R., Ellenbogen, K. A., Gold, M. R., Goldschlager, N. F., Hamilton, R. M., Joglar, J. A., Kim, R. J., Lee, R., Marine, J. E., McLeod, C. J., Oken, K. R., Patton, K. K., Pellegrini, C. N., Selzman, K. A., Thompson, A., & Varosy, P. D. (2019). 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and
Cardiac Conduction Delay. Journal of the American College of Cardiology, 74(7), e51–e156. https://doi.org/10.1016/j.jacc.2018.10.044
Farkas, J. (2021, October 1). Hypothermia. EMCrit Project. https://emcrit.org/ibcc/hypothermia/
UpToDate. (n.d.). Www.uptodate.com. Retrieved June 11, 2024, from https://www.uptodate.com/contents/accidental-hypothermia-in-adults?search=hypothermia&source=search_result&selectedTitle=1~150&usage_type=default&display_r
Näyhä, S. (2005). Environmental temperature and mortality. International Journal of Circumpolar Health, 64(5), 451–458. https://doi.org/10.3402/ijch.v64i5.18026
Decompensated Hypothyroidism (“Myxedema Coma”). (n.d.). EMCrit Project. https://emcrit.org/ibcc/myxedema/#treatment_of_cause
UpToDate. (n.d.). Www.uptodate.com. Retrieved June 11, 2024, from https://www.uptodate.com/contents/evaluation-and-management-of-elevated-intracranial-pressure-in-adults?search=increased%20intracranial%20pressure&source=search_result&selectedTitle=2~150&usage
UpToDate. (n.d.). Www.uptodate.com. https://www.uptodate.com/contents/sinus-bradycardia?search=Bradycardia&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=
Expert Commentary
Thank you for this nice review of the differential diagnosis of bradycardia. As emergency physicians, it’s easy to slip into the default of “symptomatic/unstable” vs “asymptomatic/stable” and slip past the underlying causes, and with bradycardia (as with many things), patients are often not divided quite so neatly.
Similarly, the context matters considerably: what resources are available? Is a cardiologist who can place a permanent pacer upstairs and ready with a staffed lab? Or is “definitive care” hours away (eg, requires transfer, or the cardiology team needs to come in from home). A patient with bradycardia from an acute MI will need the cath lab regardless, but in settings that require a transfer that may require transvenous pacemaker prior to transfer. On the other hand, a patient being whisked upstairs to a cath lab requires a different conversation with the cardiology team, eg preparing for transcutaneous pacing while getting the patient from the door to the balloon in a handful of minutes.
The differential can also be helpful when considering other logistics. For example, while I am happy to place a transvenous pacemaker for a patient who needs it for, say, a high degree AV block from Lyme disease, I may consider if the patient is stable enough to have a transvenous pacer placed more elegantly by the cardiology team as the patient may keep the TVP for 2 weeks but not need a permanent pacemaker.
Of course there are also secondary causes of bradycardia that need other, non-cardiac, definitive treatments, eg, overdose, hypothermia, Cushing’s response, and of course, hyperkalemia; keeping the differential in mind is part of the reason why we have not yet been replaced by robots.
Seth Trueger, MD, MPH, FACEP
Associate Professor of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kilbane, K. Power, E. (2024, Jun 17). Symptomatic Bradycardia: Considering the Differential Diagnosis. [NUEM Blog. Expert Commentary by Trueger, S]. Retrieved from http://www.nuemblog.com/blog/symptomatic-bradycardia
Other Posts You May Enjoy
Review of the ATHOS 3 trial
Written by: Saabir Kaskar, MD (NUEM ‘23) Edited by: Amanda Randolph, MD (NUEM ‘20)
Expert Commentary by: Matt McCauley, MD (NUEM’ 21)
Review of the ATHOS 3 Trial: Angiotensin II for the Treatment of Vasodilatory Shock
Angiotensin, first isolated in the late 1930s, in recent years has become the new innovative vasopressor used in intensive care units, a change driven largely by the results of the ATHOS-3 trial. The ATHOS-3 trial in 2017 explored the efficacy of angiotensin II as a vasopressor for severe vasodilatory shock. Severe shock is defined as persistent hypotension requiring vasopressors to maintain a mean arterial pressure of 65mmHg and serum lactate <2 despite adequate volume resuscitation. Two classes of vasopressors have been used in the past for hypotension. They are catecholamines and vasopressin-like peptides. The human body, however, employs a third class which is angiotensin. Angiotensin II is an octapeptide hormone and a potent vasopressor that is an integral component of the renin-angiotensin-aldosterone system. It works by activating the ANGII type 1 receptor which subsequently activates a G coupled protein pathway and phospholipase C, thereby inducing vasoconstriction.
The ATHOS-3 trial compared the efficacy and safety of angiotensin II versus placebo in catecholamine-resistant hypotension, which is defined as an inadequate response to standard doses of vasopressors. The study was designed as a phase III multicenter randomized placebo control trial taking place across 75 intensive care units in the United States from 2015 to 2017. The three main inclusion criteria were catecholamine-resistant hypotension (defined as >0.2ug/kg/min of norepinephrine or equivalent for 6-48 hours to maintain a MAP 55-70 mmHg), adequate volume resuscitation (25mL/kg of crystalloid), and features of vasodilatory shock (mixed venous O2 >70% and CVP >8mmHg or cardiac index >2.3 L/min/m2).
Patients in vasodilatory shock that met the criteria of catecholamine-resistant hypotension were randomized to treatment with angiotensin II or placebo. Angiotensin II was initiated at an infusion rate of 20ng/kg/min and adjusted during the first three hours to increase MAP to at least 75mmHg. The primary outcome of the study was the response in MAP three hours after the start of angiotensin II infusion. A response was deemed as a MAP increase of 10mmHg from baseline or a MAP over 75mmHg without an increase in baseline vasopressor infusions. During the first three hours, the angiotensin II group had a significantly greater increase in MAP than placebo (12.5mmHg vs 2.9 mmHg). Angiotensin II also allowed for rapid increases in MAP which permitted decreases in doses of baseline catecholamine vasopressor. Additionally, improvement in the cardiovascular SOFA score was significantly greater in the angiotensin II group than in the placebo group. However, the overall SOFA score did not differ between groups. Rates of adverse events such as tachyarrhythmias, distal ischemia, ventricular tachycardia, and atrial fibrillation were similar in the angiotensin II and placebo groups. Overall serious adverse events that included infectious, cardiac, respiratory, gastrointestinal, or neurologic events were reported in 60.7% of patients who received angiotensin II and 67.1% of patients who received placebo.
The strengths and limitations of the ATHOS 3 trial are critical to how its author’s conclusions should be interpreted. The strengths of the study include that it was a randomized double-blind control trial examining a new class of vasopressor for refractory vasodilatory shock. Refractory shock is a common condition with high mortality, and so the investigation of an additional treatment modality can be of great clinical impact. However, one limitation of the study was that it was underpowered to demonstrate a mortality difference. It showed improvement in blood pressure which is a clinically important parameter but not a patient-oriented outcome. Interestingly, when vasopressin was studied in 2008, it similarly did not show a mortality benefit when added to norepinephrine infusion in septic shock2. It did, however, show a decrease in norepinephrine dosing which parallels the findings of the ATHOS 3 trial.
An additional point of contention with the ATHOS 3 trial is that the manuscript does not report an increase in thrombotic risk. It has been shown that angiotensin II increases thrombin formation and impairs thrombolysis3. The FDA even reports angiotensin II has a risk for thrombosis as there was a higher incidence (13% vs 5%) of arterial and venous thrombotic events in the angiotensin II vs placebo group in the ATHOS 3 trial itself. For this reason, the FDA recommends concurrent VTE prophylaxis with the use of angiotensin II. Further data regarding the thrombotic risk of angiotensin II would be helpful to determine which patient populations the vasopressor should be avoided in.
Overall, the author’s conclusion in the ATHOS 3 trial is that angiotensin II increased blood pressure in patients with a vasodilatory shock that did not respond to high doses of conventional vasopressors. It has been shown to raise mean arterial pressure over 75 mm Hg or by an increase of 10 mm Hg within three hours. The ATHOS 3 trial, however, did not demonstrate a mortality benefit when using angiotensin II. Further studies are needed to elucidate whether Angiotensin II truly improves patient outcomes in vasodilatory shock.
Expert Commentary
Thank you for this great summary of the ATHOS 3 trial. While this trial paved the way for the clinical use of angiotensin II as a vasopressor, you’ve raised some salient points as to why we should approach this emerging intervention with skepticism. The biggest shortcoming in my mind is the primary outcome of the study; it’s not particularly impressive that a vasopressor resulted in higher blood pressures compared to a placebo. Mortality benefit is an extremely elusive goal in critical care research1 but that doesn’t discount the fact that ATHOS 3 wasn’t designed to demonstrate an improvement in any patient-oriented outcome. ICU length of stay, hospital length of stay, ventilator-dependent days, or rate of renal replacement therapy: these are all things that matter to our patients and to our health systems and they are more fruitful targets when we investigate interventions.
There’s been some study of angiotensin II in the years since it has landed in our hospital formularies and there has not been robust data supporting its use. Some of the most recent data come from a multi-center retrospective study that includes patients from Northwestern. This review of 270 patients receiving angiotensin II demonstrated that 67% of patients were able to maintain a MAP of 65 with stable or reduced vasopressor doses. Univariate analysis showed that these patients that responded did have a statistically significant mortality benefit over the patients deemed nonresponders (41% vs 25%)2. If we are going to find a benefit of this drug, further study predicting which patients will be responders is necessary but this study did note that patients already receiving vasopressin and those with lower lactates (6.5 vs 9.5) were more likely to respond. Outside of septic shock, there is interest in the use of angiotensin II in refractory vasoplegia associated with post-cardiac surgery3 and anti-hypertensive overdose4. These are, of course, only hypothesis-generating.
But what does that mean to us clinically in the ED and ICU? This data shows us that angiotensin II can make the blood pressure better but I would never let it distract you from the things we know matter in sepsis resuscitation. Source control timely antibiotics, rational fluid resuscitation, and ruling out other causes of vasopressor refractory shock to include anaphylaxis, hemorrhage, adrenal insufficiency, LVOT obstruction, and any other cause of cardiogenic shock need to be ruled out and addressed. In my personal practice, I make sure to optimize these and start vasopressin shortly after the initiation of norepinephrine. In a patient already on vaso that has stopped responding to escalating doses of norepinephrine, I reach for my ultrasound probe and reassure myself that there isn’t significant sepsis-related myocardial dysfunction because those patients may benefit from a trial of an inotrope like epinephrine. In those with a good cardiac squeeze, I think it’s appropriate to discuss with your intensivist and clinical pharmacist the utility of adding angiotensin II as part of a kitchen-sink approach. Until we have more data about the benefits of this extremely expensive intervention, I wouldn’t lose sleep if you’re unable to secure it for your patient.
References
Chawla LS et al. Intravenous Angiotensin II for the Treatment of High-Output Shock (ATHOS Trial): A Pilot Study. Crit Care 2014; 18(5): 534. PMID: 25286986
Russell JA et al. Vasopressin Versus Norepinephrine Infusion in Patients with Septic Shock. NEJM 2008; 358(9): 877 – 87. PMID: 18305265
Celi A et al. Angiotensin II, Tissue Factor and the Thrombotic Paradox of Hypertension. Expert Review of Cardiovascular Therapy 2010; 8(12): 1723-9 PMID: 21108554
Santacruz CA, Pereira AJ, Celis E, Vincent JL. Which Multicenter Randomized Controlled Trials in Critical Care Medicine Have Shown Reduced Mortality? A Systematic Review. Crit Care Med. 2019;47(12):1680-1691. doi:10.1097/CCM.0000000000004000
Wieruszewski PM, Wittwer ED, Kashani KB, et al. Angiotensin II Infusion for Shock: A Multicenter Study of Postmarketing Use. Chest. 2021;159(2):596-605. doi:10.1016/j.chest.2020.08.2074
Papazisi O, Palmen M, Danser AHJ. The Use of Angiotensin II for the Treatment of Post-cardiopulmonary Bypass Vasoplegia. Cardiovasc Drugs Ther. Published online October 21, 2020. doi:10.1007/s10557-020-07098-3
Carpenter JE, Murray BP, Saghafi R, et al. Successful Treatment of Antihypertensive Overdose Using Intravenous Angiotensin II. J Emerg Med. 2019;57(3):339-344. doi:10.1016/j.jemermed.2019.05.027
Matt McCauley, MD
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kaskar, S. Randolph, A. (2022, Feb 14). Review of ATHOS 3 trial. [NUEM Blog. Expert Commentary by McCauley, M]. Retrieved from http://www.nuemblog.com/blog/review-athos3-trial.
Other Posts You May Enjoy
SonoPro Tips and Tricks for Aortic Aneurysm and Dissection
Written by: John Li, MD (NUEM ‘24) Edited by: Andra Farcas, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Aortic ultrasound is a staple in emergency point of care ultrasound. It has incredible sensitivity (97.5-100%) and specificity (94.1-100%) in detecting abdominal aortic aneurysms and can provide a diagnosis for critically ill patients in seconds. [1-4] However, it can often be a technically difficult study for beginner sonographers due to shadowing bowel gas and patient body habitus. Follow along in this installment of our Sono Pro Tips and Tricks Series to become an expert in finding aortas!
Beyond the classic elderly male smoker with abdominal, flank, or back pain, what are other scenarios where you would use aortic ultrasound?
Older patients with limb ischemia - an aortic aneurysm can have atherosclerosis or a mural thrombus which can embolize and cause an arterial occlusion!
“But they fixed my aorta!” Aortic endograft leakage can sometimes present with symptoms that are similar to a AAA rupture, such as back pain, flank pain, or hemodynamic instability.
How to scan like a Pro
Always Start Smart: Aortic ultrasound can be tricky because of factors that seem out of our control, such as bowel gas or patient body habitus.
When scanning for an abdominal aortic aneurysm, start scanning in the epigastric region with a transverse view and apply constant pressure, gently pushing the bowel gas out of the way as you slide the probe down towards the patient’s feet.
Tell your patients to bend their knees! This relaxes the abdominal musculature and can help you move bowel gas or make better contact with the probe.
What if you still can’t see it? Try looking in the right upper quadrant view of the FAST exam!
Start with your probe in the right mix-axillary line and use the liver as your acoustic window. You may need to fan anteriorly or posteriorly depending on the patient’s body habitus and your positioning.
Unfortunately, this view predominantly visualizes the superior aspect of the abdominal aorta, and it can be difficult to visualize the inferior abdominal aorta or the bifurcation.
Here we are looking at a modified RUQ view, where the aorta is visualized on the bottom part of the screen using the liver as an acoustic window. (acep.org)
Pro Pickups!
What’s that weird aneurysm?
Most people are familiar with the classic fusiform aortic aneurysm, but saccular aneurysms can be easily missed because of shadowing bowel gas obstructing parts of the aorta. Saccular aneurysms actually have a higher risk of rupture and repair is recommended for smaller diameters.
Here you can see two images in the longitudinal axis of the different kinds of abdominal aortic aneurysms. On the left is a saccular aneurysm and on the right is a fusiform one. Be sure to pay attention to the mural thrombus in the walls of both of these aortas - they can embolize and cause arterial occlusions! (med.emory.edu)
2. How big is that aorta anyways?
Be sure to always measure the aorta from outside wall to outside wall!
Many aortic aneurysms have a mural thrombus or intraluminal clot, and it can be very easy to mistake these for extra-luminal contents.
Remember the concerning numbers: >5.5cm for men and >5cm for women!
What the Pros Do Next
Abdominal Aortic Aneurysm
If the patient is hemodynamically unstable (defined as BP <90/60, altered mental status, or other signs of end-organ damage), go straight to the OR!
If the patient is hemodynamically stable (defined as the absence of any of the above), then the next step is to obtain further imaging, such as a CT Angiogram, which is the imaging gold standard.
If you are concerned about a large AAA that could be a contained leak but the patient is hemodynamically stable, then we recommend an emergent vascular surgery consult
If you find a small AAA (defined as <5cm in women or <5.5cm in men) that you do not think is actively contributing to the patient’s symptoms, then we recommend outpatient vascular surgery follow up
SonoPro Tips - Where to Learn More
Do you want to review more examples of pathologic images that you may see when you are doing an aortic ultrasound? Be sure to check out The Pocus Atlas by our expert editor Dr. Macias. Aortic pathology is quite rare, and going through these images will help immensely in recognizing this diagnosis in emergent situations. If you’re interested in looking at some of the evidence behind aortic ultrasound, be sure to check out the evidence atlas here as well.
References
Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013 Feb;20(2):128-38. doi: 10.1111/acem.12080. PMID: 23406071.
Hunter-Behrend, Michelle, and Laleh Gharahbaghian. “American College of Emergency Physicians.” ACEP // Home Page, 2016, www.acep.org/how-we-serve/sections/emergency-ultrasound/news/february-2016/tips-and-tricks-big-red---the-aorta-and-how-to-improve-your-image/.
Ma, John, et al. Ma and Mateer's Emergency Ultrasound. McGraw-Hill Education, 2020.
Mallin, Mike, and Matthew Dawson. Introduction to Bedside Ultrasound: Volume 1. Emergency Ultrasound Solutions, 2013.
Macias, Michael. TPA, www.thepocusatlas.com/.
Expert Commentary
Another great Sono Pro Post! Thank you John Li and Andra for helping everyone move from good to great when scanning for Abdominal Aortic Aneurysms. As noted, this application defines Emergency Ultrasound as a fast (pun intended), accurate, and life saving diagnostic tool for every EM physicians tool belt. When consistent probe pressure does not do the trick, consider the RUQ view for a quick look. Since most AAA’s are fusiform, this may quickly confirm your suspicions and prompt the call to get the OR ready. Be sure to visualize the entire abdominal aorta throughout in both short and long axis to identify saccular aneurysms and even the rare aortic occlusion!
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Li, J. Farcas, A. (2021 Oct 11). SonoPro Tips and Tricks for Aortic Aneurysm. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-aortic-aneurysm
Other Posts You May Enjoy
SonoPro Tips and Tricks for Pulmonary Embolism
Written by: Megan Chenworth, MD (NUEM ‘24) Edited by: Abiye Ibiebele, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Did you know that focused transthoracic cardiac ultrasound (FOCUS) can help identify PE in tachycardic or hypotensive patients? (It has been shown to have a sensitivity of 92% for PE in patients with an HR>100 or SBP<90, and approaches 100% sensitivity in patients with an HR>110 [1]). Have a hemodynamically stable patient with PE and wondering how to risk stratify? FOCUS can identify right heart strain better than biomarkers or CT [2].
Who to FOCUS on?
Patients presenting with chest pain or dyspnea without a clear explanation, or with a clinical concern for PE. The classic scenario is a patient with pleuritic chest pain with VTE risk factors such as recent travel or surgery, systemic hormones, unilateral leg swelling, personal or family history of blood clots, or known hypercoagulable state (cancer, pregnancy, rheumatologic conditions).
Patients presenting with unexplained tachycardia or dyspnea with VTE risk factors
Unstable patients with undifferentiated shock
When PE is suspected but CT is not feasible: such as when the patient is too hemodynamically unstable to be moved to the scanner, too morbidly obese to fit on the scanner, or in resource-limited settings where scanners aren’t available
One may argue AKI would be another example of when CT is not feasible (though there is some debate over the risk of true contrast nephropathy - that is a discussion for another blog post!)
How to scan like a Pro
Key is to have the patient as supine as possible - this may be difficult in truly dyspneic patients
If difficulty obtaining views arise, the left lateral decubitus position helps bring the heart closer to the chest wall
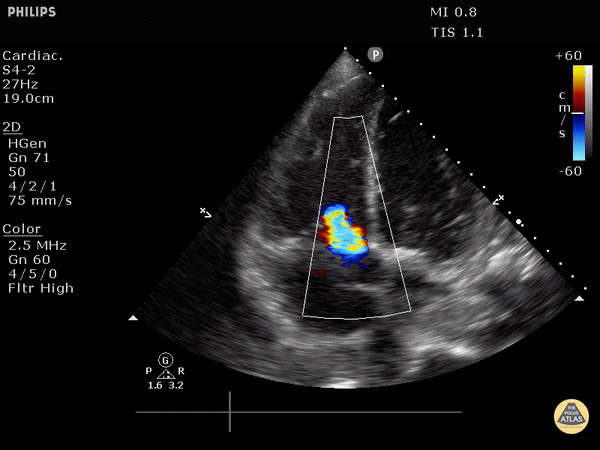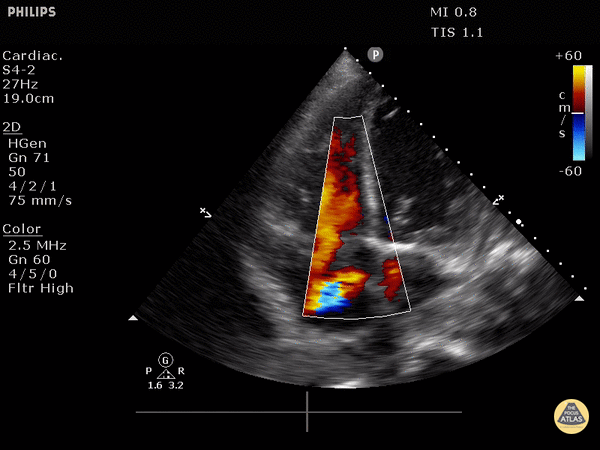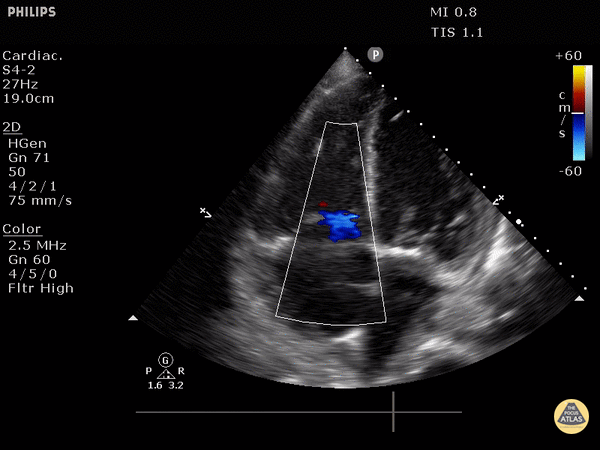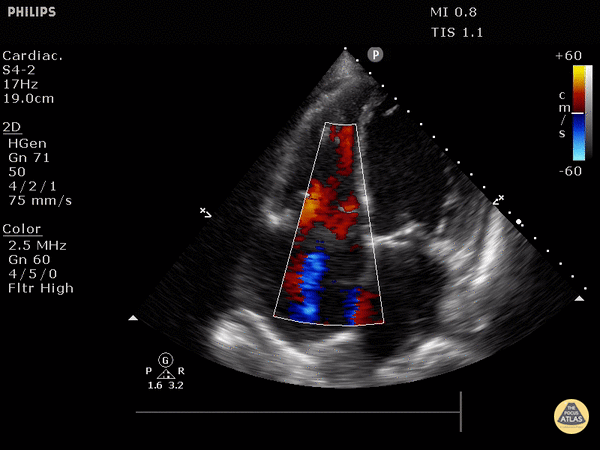
FOCUS on these findings
You only need one to indicate the presence of right heart strain (RHS).
Right ventricular dilation
Septal flattening: Highly specific for PE (93%) in patients with tachycardia (HR>100) or hypotension (SBP<90) [1]
Tricuspid valve regurgitation
McConnell’s sign
Definition: Akinesis of mid free wall and hypercontractility of apical wall (example below)
The most specific component of FOCUS: 99% specific for patients with HR>100bpm or SBP<90 [1]
Tricuspid annular plane systolic excursion (TAPSE)
The most sensitive single component of FOCUS: TASPE < 2cm is 88% sensitive for PE in tachycardic and hypotensive patients; 93% sensitive when HR > 110 [1]
Where to FOCUS
Apical 4 Chamber (A4C) view: your best shot at seeing it all
Find the A4C view in the 5th intercostal space in the midclavicular line
Optimize your image by sliding up or down rib spaces, sliding more lateral towards the anterior axillary line until you see the apex with the classic 4 chambers - if the TV and MV are out of the plane, rotate the probe until you can see both openings in the same image; if the apex is not in the middle of the screen, slide the probe until the apex is in the middle of the screen. If you are having difficulty with this view, position the patient in the left lateral decubitus.
Important findings:
RV dilation: the normal RV: LV ratio in diastole is 0.6:1. If the RV > LV, it is abnormal. (see in the image below)
Septal flattening/bowing is best seen in this view
McConnell’s sign: akinesis of the free wall with preserved apical contractility
McConnell’s Sign showing akinesis of the free wall with preserved apical contractility
4. Tricuspid regurgitation can be seen with color flow doppler when positioned over the tricuspid valve
Tricuspid regurgitation seen with color doppler flow
5. TAPSE
Only quantitative measurement in FOCUS, making it the least user-dependent measurement of right heart strain [3]
A quantitative measure of how well the RV is squeezing. RV squeeze normally causes the tricuspid annulus to move towards the apex.
Fan to bring the RV as close to the center of the screen as possible
Using M-mode, position the cursor over the lateral tricuspid annulus (as below)
Activate M-mode, obtaining an image as below
Measure from peak to trough of the tracing of the lateral tricuspid annulus
Normal >2cm
How to measure TAPSE using ultrasound
Parasternal long axis (PSLA) view - a good second option if you can’t get A4C
Find the PSLA view in the 4th intercostal space along the sternal border
Optimize your image by sliding up, down, or move laterally through a rib space, by rocking your probe towards or away from the sternum, and by rotating your probe to get all aspects of the anatomy in the plane. The aortic valve and mitral valve should be in plane with each other.
Important findings:
RV dilation: the RV should be roughly the same size as the aorta and LA in this view with a 1:1:1 ratio. If RV>Ao/LA, this indicates RHS.
Septal flattening/bowing of the septum into the LV (though more likely seen in PSSA or A4C views)
Right heart strain demonstrated by right ventricle dilation
Parasternal Short Axis (PSSA) view: the second half of PSLA
Starting in the PSLA view, rotate your probe clockwise by 90 degrees to get PSSA
Optimize your image by fanning through the heart to find the papillary muscles - both papillary muscles should be in-plane - if they are not, rotate your probe to bring them both into view at the same time
Important findings:
Septal flattening/bowing: in PSSA, it is called the “D-sign”.
“D-sign” seen on parasternal short axis view. The LV looks like a “D” in this view, particularly in diastole.
Subxiphoid view: can add extra info to the FOCUS
Start just below the xiphoid process, pointing the probe up and towards the patient’s left shoulder
Optimize your image by sliding towards the patient’s right, using the liver as an echogenic window; rotate your probe so both MV and TV are in view in the same image
Important findings
Can see plethoric IVC if you fan down to IVC from RA (not part of FOCUS; it is sensitive but not specific to PE)
Plethoric IVC that is sensitive to PE
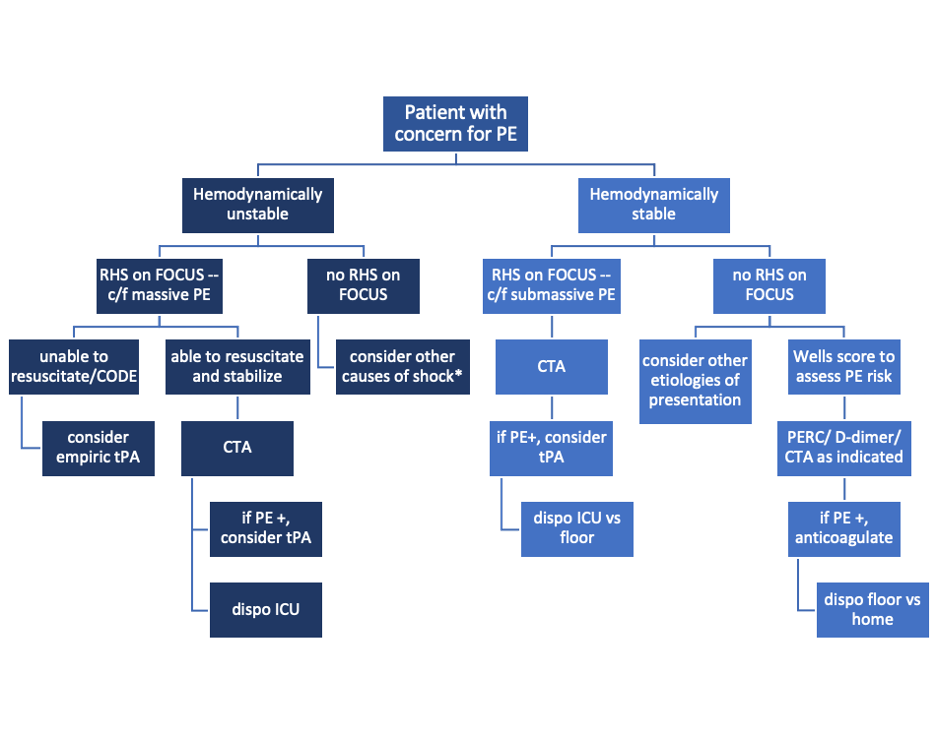
What to do next?
Sample algorithm for using FOCUS to assess patients with possible PE.
*cannot completely rule out PE, but negative FOCUS makes PE less likely
Limitations to keep in mind:
FOCUS is great at finding heart strain, but the lack of right heart strain does not rule out a pulmonary embolism
Systematic review and meta-analysis concluded that the overall sensitivity of FOCUS for PE is 53% (95% CI 45-61%) for all-comers [5]
Total FOCUS exam requires adequate PSLA, PSSA, and A4C views – be careful when interpreting inadequate scans
Can see similar findings in chronic RHS (pHTN, RHF)
Global thickening of RV (>5mm) can help distinguish chronic from acute RHS
McConell’’s sign is also highly specific for acute RHS, whereas chronic RV failure typically appears globally akinetic/hypokinetic
SonoPro Tips - Where to Learn More
Right Heart Strain at 5-Minute Sono: http://5minsono.com/rhs/
Ultrasound GEL for Sono Evidence: https://www.ultrasoundgel.org/posts/EJHu_SYvE4oBT4igNHGBrg, https://www.ultrasoundgel.org/posts/OOWIk1H2dePzf_behpaf-Q
The Pocus Atlas for real examples: https://www.thepocusatlas.com/echocardiography-2
The Evidence Atlas for Sono Evidence: https://www.thepocusatlas.com/ea-echo
References
Daley JI, Dwyer KH, Grunwald Z, Shaw DL, Stone MB, Schick A, Vrablik M, Kennedy Hall M, Hall J, Liteplo AS, Haney RM, Hun N, Liu R, Moore CL. Increased Sensitivity of Focused Cardiac Ultrasound for Pulmonary Embolism in Emergency Department Patients With Abnormal Vital Signs. Acad Emerg Med. 2019 Nov;26(11):1211-1220. doi: 10.1111/acem.13774. Epub 2019 Sep 27. PMID: 31562679.
Weekes AJ, Thacker G, Troha D, Johnson AK, Chanler-Berat J, Norton HJ, Runyon M. Diagnostic Accuracy of Right Ventricular Dysfunction Markers in Normotensive Emergency Department Patients With Acute Pulmonary Embolism. Ann Emerg Med. 2016 Sep;68(3):277-91. doi: 10.1016/j.annemergmed.2016.01.027. Epub 2016 Mar 11. PMID: 26973178.
Kopecna D, Briongos S, Castillo H, Moreno C, Recio M, Navas P, Lobo JL, Alonso-Gomez A, Obieta-Fresnedo I, Fernández-Golfin C, Zamorano JL, Jiménez D; PROTECT investigators. Interobserver reliability of echocardiography for prognostication of normotensive patients with pulmonary embolism. Cardiovasc Ultrasound. 2014 Aug 4;12:29. doi: 10.1186/1476-7120-12-29. PMID: 25092465; PMCID: PMC4126908.
Hugues T, Gibelin PP. Assessment of right ventricular function using echocardiographic speckle tracking of the tricuspid annular motion: comparison with cardiac magnetic resonance. Echocardiography. 2012 Mar;29(3):375; author reply 376. doi: 10.1111/j.1540-8175.2011.01625_1.x. PMID: 22432648.
Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta‐analysis. J Am Soc Echocardiogr 2017;30:714–23.e4.
Expert Commentary
RV function is a frequently overlooked area on POCUS. Excellent post by Megan looking specifically at RV to identify hemodynamically significant PEs. We typically center our image around the LV, so pay particular attention to adjust your views so the RV is optimized. This may mean moving the footprint more laterally and angle more to the patient’s right on the A4C view. RV: LV ratio is often the first thing you will notice. When looking for a D-ring sign, make sure your PSSA is actually in the true short axis, as a diagonal cross-section may give you a false D-ring sign. TAPSE is a great surrogate for RV systolic function as RV contracts longitudinally. Many patients with pulmonary HTN or advanced chronic lung disease can have chronic RV failure, lack of global RV thickening. Lastly remember, that a positive McConnell’s sign is a great way to distinguish acute RHS from chronic RV failure.
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Chenworth, M. Ibiebele, A. (2021 Oct 4). SonoPro Tips and Tricks for Pulmonary Embolism. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-pulmonary-embolism
Other Posts You May Enjoy
Cocaine Chest Pain
Written by: Maren Leibowitz, MD (NUEM ‘23) Edited by: Zach Schmitz, MD (NUEM ‘21) Expert Commentary by: David Farman, MD
Expert Commentary
Cocaine chest pain was something that was frequently discussed but rarely encountered during my training and during my 11 years practicing in suburban and metropolitan Indiana (we're more of a heroin/meth state). I suspect there is significant regional and local variation in the epidemiology of cocaine chest pain, likely influenced by the economics and local availability or popularity of intoxicants.
When cocaine is more expensive, we tend to see more methamphetamine use. And vice-versa. I was once told by a local sheriff that "people will get high on whatever they can get for less than $20" and I have found that to be true in practice. I wouldn't expect that an emergency physician would be intimately familiar with the local micro-economics of the drug trade, but one should expect there to be a periodic waxing/waning of cocaine chest pain presentations. Similarly, it may be a more frequent complaint dependent on cocaine's local popularity and availability.
When consulting with a Cardiologist about a cocaine chest pain case, it is important for the emergency physician to avoid letting premature closure or psychosocial biases unduly influence the patient's disposition. It is not unheard of for physicians to minimize objective findings (ST segment abnormalities or biomarker elevation) and attribute them to the vasoactive properties of cocaine. I have certainly been tempted to do so myself. However, the article wisely points out the physiologic changes induced by cocaine use, both acutely and chronically. Platelet aggregation and atherogenesis can absolutely promote a scenario that would require PCI in even the most frequent of 'frequent fliers' with cocaine chest pain.
In short, I would have a low threshold to involve Cardiology in a patient who has objective findings regardless of their use of cocaine. Similarly, a Cardiology request for a Urine Drug Screen shouldn't delay a patient's trip to the cath lab if they have a STEMI. An exception to this may be a patient who has had recent coronary angiography that objectively demonstrates normal coronaries. In that scenario I would consider serial markers, conservative management and strong consideration of non-cardiac causes of the pain (dissection, pneumothorax, etc.).
David Farman, MD FACEP
Emergency Medicine Physician
Franciscan Health Lafayette East
How To Cite This Post:
[Peer-Reviewed, Web Publication] Leibowitz, M. Schmitz, Z. (2021, May 10). Cocaine Chest Paine. [NUEM Blog. Expert Commentary by Farman, D]. Retrieved from http://www.nuemblog.com/blog/cocaine-chest-pain
Other Posts You May Enjoy
Apple Heart Study
Written by: Em Wessling, MD (NUEM ‘22) Edited by: Dana Loke, MD (NUEM ‘19) Expert Commentary by: Rod Passman, MD
Chief Complaint: My watch thinks I have Atrial Fibrillation!
As technology advances, medicine must continue to advance in pace. Wearable technology has been evolving for decades. The information gathered from a wide range of these devices may someday help to provide healthcare workers with valuable information about a patient’s condition. However, for now, there is limited research on their utility within the healthcare field.
Thus far, both Apple Watch and Fitbit have been shown to correctly identify tachycardia during atrial tachyarrhythmias, but their accuracy to the heart rate varied with the type of arrhythmia (1,2). Apple Watch has been shown to be more accurate than Fitbit (1,2). The WATCH AF trial demonstrated it was possible with reasonable sensitivity (93.7%) and specificity (98.2) to use smart watches to diagnose Atrial Fibrillation (3).
How Apple Watch is tracking atrial fibrillation:
- Photoplethysmography: the use of light to determine volume within a structure at a given time
- Pulse is estimated by time between peak volume seen by photoplethysmography.
- When the pulse is highly variable between consecutive beats, irregular heart beat is suspected.
Apple Heart Study: The plan and the preliminary data (4, 5)
Study Design: Prospective, single arm pragmatic study
- Enrolled 419,093 participant
- Inclusion Criteria: appropriate Apple technology, Age≥22 years, US resident, proficient in English, valid phone number and email.
- Exclusion criteria: self-reported atrial fibrillation , atrial flutter, or anticoagulation
- Methods: “Irregular Pulse Notification” (indication of possible atrial fibrillation) sent to participants if 5/6 irregular pulses within 48 hour period, at which point participant was instructed to wear EKG patch for up to 7 days.
- Primary Outcome: Proportion of patients alerted with “Irregular Pulse Notification” who were found to have atrial fibrillation or atrial flutter on EKG patch, in the 65+ population as well as in all-comers.
- Secondary Outcomes: Positive predictive value (PPV) of irregular heart rhythm notification; percentage of those with irregular notification who contacted a health care professional within 3 months.
Preliminary Data presented at ACC:
- Participants who received “Irregular Pulse Notification”: 2,161 (0.52% all comers)
- Participants age >65 who received “Irregular Pulse Notification”: >3%
- EKG Patches sent to 658 participants; 450 returned.
34% of those returned showed atrial fibrillation
PPV for Tachogram: 71%
PPV for “Irregular Pulse Notification”: 84%
- Notification to doctor - approx. 50%
Limitations:
Small sample size for EKG patches, despite high enrollment
Self-reported data
Self-selecting group, i.e.may not be able to extrapolate prevalence data to those who do not wear smart watches
Potential Impact on Emergency Departments:
As more and more studies validate the accuracy of wearable technology to measure and recognize health conditions, the implications must be analyzed as well.
Prior to 2017, researchers began to predict that there would be an expansive increase in the rates of atrial fibrillation due to “worldwide aging” (6). While this review acknowledged there were “potential applications” for smart phone technology in the diagnosis, their predictions of the expanse of the epidemic of atrial fibrillation preceded definitive research showing increased diagnosis rates with wearable technology, which will likely only further expedite this growing patient population. The mSToPS Trial showed that immediate in-home monitoring with an EKG patch had 3% greater rates of atrial fibrillation diagnosis compared to delayed EKG monitoring at 4 months. This led to increased use of anticoagulants and increased health care utilization (7). If this increase was seen with EKG patches, consider the influx of patients to primary care and cardiology clinics in addition to emergency departments that can be projected based on the rise of smart watch detection of atrial fibrillation. Researchers in Australia had begun studying this prior to the commencement of the Apple Heart Study (8). When cardiac patients were asked if they trusted smart watches to predict arrhythmia and measure their heart rate only 53% agreed; however, that did not stop 91% from reporting they would seek care if their watch alerted them about an abnormality (8). While the preliminary data from the Apple Heart study shows that a much smaller percentage of those who were not previously cardiac patients sought medical care when alerted by their Apple Watch, further study is needed to see the extent to which advances in smart watch health technology will lead to an influx in patients to the Emergency Department due to concerns of arrhythmia found by a smartwatch (5).
While the accuracy of these methods of arrhythmia detection are still being studied, the potential for ED presentation with this chief complaint will continue to rise. In the fourth quarter of 2017 financial year, Apple alone sold greater than 8 million smart watches worldwide, making it the largest watch vender in the world (9). With these increased sales, comes the potential for increased recognition of arrythmia by smartwatch. Healthcare organizations throughout the country must strive to develop effective and efficient clinical pathways in order to evaluate, potentially diagnose, and treat this patient population. Upon presentation to the Emergency Department, each patient should receive an EKG, telemetry monitoring while in the Emergency Department and screaming lab work: often including CBC, BMP + Mg, and troponin. From there, the pathway may vary. Many would agree, if the patient is and has always been asymptomatic, work up is unremarkable, with normal sinus rhythm on their EKG, discharge home with an EKG patch and follow up with cardiology is reasonable. Conversely, an EKG showing atrial fibrillation would constitute a new diagnosis and further work up would proceed as with any other new diagnosis of Atrial Fibrillation. However, for those who fall in-between, the disposition is not as clear. What would you do?
Expert Commentary
More than 800 years ago, Maimonides described an irregular pulse that likely represented atrial fibrillation (AF). The development of the electrocardiogram by Einthoven 700 years later allowed surface recordings of human AF for the first time.1 With the recognition that AF is often asymptomatic and paroxysmal, the development of inexpensive, non-invasive, passive monitors for irregular rhythm identification has long been recognized as a potentially important tool for arrhythmia detection and management.
At its core (pun intended), the purpose of the Apple Heart Study was to assess the feasibility of AF screening in large populations by monitoring participants with a wrist-worn photoplethysmography (PPG) monitor.2 The PPG algorithm in the Apple Watch samples the pulse several times daily during periods of physical inactivity and increases the sampling rate if an irregular tachogram is detected. If 5 out of 6 tachograms are consistent with AF (requiring > 60 minutes of AF), the wearer receives an irregular rhythm notification. Since the version of the Apple Watch used in the study did not have the 30-second ECG feature (available in Series 4 watches and later), the Apple Heart Study protocol asked those who received the irregular rhythm notification to wear an ECG patch at a later date.
Several important facts can be gleaned from the Apple Heart Study. First, the study virtually enrolled 419,297 individuals in less than a year, a testament to the interest in the subject matter, the ease of remote enrollment when appropriate, and the enormous potential of digital health studies. Second, the fear that the healthcare system would be inundated with false positive AF notifications appears unfounded as 99.8% of participants under age 40 did not receive an irregular rhythm notification. Third, the positive predictive value for the irregular rhythm notification was surprisingly high (84%) despite that fact that the patch was applied a mean of 13 days following the notification and was worn for less than 7 days on average. This last point is worth emphasizing since with paroxysmal AF, a negative monitor placed two weeks after an irregular rhythm notification may simply mean that AF was not present during both time periods.
The study also has some important caveats. The Apple Heart Study did not report the sensitivity and specificity of the PPG algorithm for AF detection, a critical piece of missing data needed for clinical care and future research. Furthermore, only a minority of patients who received an irregular rhythm notification actually wore and returned the ECG monitor, showing that virtual enrollment doesn’t always translate into virtual protocol compliance. From a research perspective, wearable AF monitors have allowed for large-scale screening studies such as the Huawei Heart and Heartline Studies aimed at understanding the true prevalence of AF and the risks and benefits of early detection and treatment.3,4 From a clinical perspective, a patient who says “my watch says I have AF” still requires ECG confirmation, but that too has been made easier with the new generation of wearables.
References
1. Prystowsky EN. The history of atrial fibrillation: the last 100 years. J Cardiovasc Electrophysiol. 2008;19(6):575-582. doi:10.1111/j.1540-8167.2008.01184.
2. Perez MV, Mahaffey KW, Hedlin H, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381(20):1909-1917. doi:10.1056/NEJMoa1901183
3. Guo Y, Wang H, Zhang H, et al. Mobile Photoplethysmographic Technology to Detect Atrial Fibrillation. J Am Coll Cardiol. 2019;74(19):2365-2375. doi:10.1016/j.jacc.2019.08.019
4. www.heartline.com
Rod Passman, MD
Professor, Feinberg School of Medicine
Cardiac Electrophysiology
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Wessling, E. Loke, D. (2021, Jan 25). Apple Heart Study. [NUEM Blog. Expert Commentary by Passman, R]. Retrieved from http://www.nuemblog.com/apple-heart.
Other Posts You May Enjoy
References
1. Koshy, Anoop N., et al. "Smart watches for heart rate assessment in atrial arrhythmias." International journal of cardiology 266 (2018): 124-127.
2. Koshy, A., et al. "Heart Rate Assessment by Smart Watch: Utility or Futility?." Heart, Lung and Circulation 26 (2017): S280-S281.
3. Dörr, Marcus, et al. "The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation." JACC: Clinical Electrophysiology5.2 (2019): 199-208.
4. Turakhia, Mintu P., et al. "Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study." American heart journal207 (2019): 66-75.
5. ACC News Story. “Apple Heart Study Identifies AFib in Small Group of Apple Watch Wearers.” American College of Cardiology: Latest in Cardiology, American College of Cardiology, 16 Mar. 2019, www.acc.org/latest-in-cardiology/articles/2019/03/08/15/32/sat-9am-apple-heart-study-acc-2019.
6. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195–203. doi:10.11909/j.issn.1671-5411.2017.03.011
7. Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. JAMA.2018;320(2):146–155.
8. Koshy, A., et al. "Cardiac Patients Likely to Seek Medical Assistance Based on Abnormal Heart Rate Readings on Smart Watches or Smartphone ECG Monitors." Heart, Lung and Circulation 26 (2017): S280.
9. Canalys Press Team. “18 Million Apple Watches Ship in 2017, up 54% on 2016.” Canalys Newsroom, Canalys, 6 Feb. 2018, www.canalys.com/newsroom/18-million-apple-watches-ship-2017-54-2016.
A “Pill-in-the-Pocket” Approach to Paroxysmal Atrial Fibrillation
Written by: David Feiger, MD (NUEM ‘22) Edited by: Jon Andereck, MD, MBA NUEM ‘19) Expert Commentary by: Kaustubha Patil, MD
The Case
A healthy 65-year-old male with paroxysmal atrial fibrillation presents to the emergency department in atrial fibrillation with rapid ventricular rate. His blood pressure is 135/83, heart rate 135, respirations 15 with an O2 saturation of 98% on room air. He states that he took his “pill-in-the-pocket” four hours prior to presentation and his symptoms did not resolve.
Atrial fibrillation
A study in the Western Journal of Emergency Medicine in 2013 observed the costs associated with emergency department (ED) treatment and discharge of patients presenting with atrial fibrillation (AF) or atrial flutter was $5,460 [10]. Those admitted to the hospital naturally incur far higher costs. For those eligible, a visit to the ED could be avoided with the “pill-in-the-pocket” approach.
What is the “pill-in-the-pocket” approach?
The “pill-in-the-pocket” approach is the administration of a prescribed class IC antiarrhythmic, either flecainide or propafenone, following recent onset of episodes of palpitations in patients with paroxysmal AF. It is generally initiated by the patient’s cardiologist after extensive cardiac evaluation to rule out structural disease and other conduction abnormalities. The idea is to terminate the suspected episode of AF without having to present to an ED or clinic. Several studies have investigated the safety of this approach and supported this method of outside-the-hospital termination of paroxysmal AF events [2, 14].
Who is eligible for the “pill-in-the-pocket” approach?
In a study in the New England Journal of Medicine supporting the feasibility and safety of this out-of-hospital treatment, only specific patients were selected to participate. Inclusion criteria included:
healthier patients between 18 and 75 years old
a history of infrequent AF not associated with chest pain, hemodynamic instability, dyspnea, or syncope
no significant electrocardiographic abnormalities (pre-excitations, bundle branch blocks, long QT interval, etc.)
no structural or functional cardiac diseases
no history of thromboembolic episodes
no current use of an antiarrhythmic medication
not currently pregnant
no significant chronic disease including but not limited to muscular dystrophies, systemic collagen disease, and renal or hepatic insufficiency
These patients were then admitted to the hospital for a cardiac workup and were trialed on either flecainide or propafenone with successful pharmacologic cardioversion in the inpatient setting. Both flecainide and propafenone are proarrhythmic, thus structural heart diseases must be ruled out before their use and patients should be monitored during initiation of therapy [2].
How do flecainide and propafenone work?
Flecainide and propafenone are both powerful class IC antiarrhythmics that strongly bind fast sodium channels with a slower association and dissociation than other class I antiarrhythmics. These drugs slow phase 0 during sodium-dependent depolarization in cardiac muscle cells of the atrial and ventricular myocardium (Figure 1). This effect is primarily important in prolonging atrial refractoriness, thus aiding in the conversion and termination of AF. Flecainide’s use in tachyarrhythmias comes from its rate-dependence property in which its efficacy is greater at faster heart rates. Propafenone has additional beta blocker activity which may enhance its overall clinical effectiveness in treating tachyarrhythmias [3, 5, 6, 13].
What are treatment options for patients presenting to the ED in AF?
For all comers presenting in AF with rapid ventricular rate to the ED, the literature has not elicited a perfect treatment modality, and no distinction is made for patients on the “pill-in-the-pocket” approach prior to arrival. Despite this, general practice guidelines are highlighted in many textbooks.
In hemodynamically-stable patients, rate control in the ED is the generally the treatment of choice. Diltiazem is often preferred as compared to beta blockers like metoprolol, which may cause hemodynamic instability in patients with underlying heart or lung disease. In otherwise healthy patients, metoprolol is a reasonable choice [1]. Digoxin is also appropriate, but onset takes several hours and is inferior to beta-blockers for rate control within 6 hours of treatment [11].
Patients who have been in AF for > 48 hours are at a greater risk of new intracardiac thrombus formation and cardioversion-induced embolization. Newer data from a study in 2014 suggests that there is an increased risk of thrombus formation with > 12 hours of AF [8] though the original guidelines for electric cardioversion within 48 hours of symptom onset have not changed. Patients who are hemodynamically stable who have been in AF for > 48 hours (and considered if > 12 hours) should be admitted from the ED for transesophageal echo to rule out intracardiac thrombus prior to cardioversion, or alternatively for initiation of anticoagulation [7].
In hemodynamically unstable patients, electrical cardioversion should be pursued regardless of a patient’s anticoagulation status [7].
Are there any treatment considerations in the ED for patients in AF taking flecainide or propafenone?
Treatment failure to the “pill-in-the-pocket” approach may be a marker of progression of the patient’s clinical disease. However, if a patient presents within an hour or two of taking their “pill-in-the-pocket,” remember the four to six-hour onset of these medications suggests they may convert during their ED stay. As in the case initially presented, the patient spontaneously converted while waiting for a provider. For those that do not, these patients warrant evaluation for new structural cardiac disease and may no longer benefit from the “pill-in-the-pocket” approach and may require daily maintenance prophylactic therapy [2].
A subset of stable patients presenting to the ED with AF with rapid ventricular rate may be taking flecainide or propafenone as maintenance therapy and not as part of the “pill-in-the-pocket” approach. In this instance, some literature has suggested that these patients can take an extra dose or two up to the maximum daily dose of flecainide (400mg) or propafenone (900mg for immediate release and 850mg for sustained release) to attempt pharmacological conversion, and it would be reasonable to attempt this in the ED [9].
To admit or not admit, that is the question.
The patient’s clinical picture should guide the provider as to the patient’s disposition. A patient’s comorbidities, current stability following conversion to normal sinus rhythm, plan for possible ablation, necessity for starting anticoagulation or maintenance medication, and means for close cardiology or PCP follow up on an outpatient basis should be factored when dispositioning the patient. Certainly, if a patient is requiring continuous IV infusion of rate controlling medications or has poor rate control, he or she should be admitted to the hospital [1]. Recent literature suggests that discharging stable patients home is safe following successful electrical, pharmacologic, or spontaneous cardioversion in the ED [4].
Final Thoughts
The “pill-in-the-pocket” approach is a great way for eligible patients to self-terminate episodes of AF in the comfort of their home, potentially preventing a costly and lengthy ED visit. While this approach has been shown to be a safe and effective for terminating paroxysmal AF, there is a significant lack of data on how to treat these patients who do not respond to these medications at home. General principals should be followed–electric cardioversion if the patient is hemodynamically unstable and rate control medications if the patient is hemodynamically stable (or rhythm control if you happen to practice in Canada [14]. Patients may be discharged home with close cardiology or PCP follow up if successfully cardioverted.
Expert Commentary
Atrial fibrillation (AF) is the most common cardiac arrhythmia and worldwide prevalence and incidence are increasing.1 It is estimated that by 2050 more than 12 million Americans will suffer from this debilitating and dangerous arrhythmia.1-2 AF presentations to Emergency Departments are certainly not without cost and the overall burden on the healthcare system will undoubtedly increase as the prevalence of atrial fibrillation continues to rise. A “pill in the pocket” approach for treatment of symptomatic atrial fibrillation has been well-described.
Class IC (sodium channel blockers) antiarrhythmic drugs (flecainide and propafenone) are the drugs of choice for “pill in the pocket” chemical cardioversion of symptomatic atrial fibrillation. There are some important considerations for this approach to be safe and effective:
The patient should have a history of infrequent paroxysmal atrial fibrillation, not persistent atrial fibrillation (episodes of AF that last greater than 7 days).
We reserve this approach for patients with symptomatic atrial fibrillation (palpitations, mild dyspnea, or mild lightheadedness) with rapid ventricular rates who do not experience dangerous symptoms such as chest pain or syncope.
As anti-arrhythmic drugs can also be pro-arrhythmic, we do not recommend Class IC antiarrhythmic drugs in patients with known structural heart disease, reduced left ventricular systolic function, or known coronary artery disease, due to the increased risk of inducing dangerous arrhythmias.
In patients who are not on therapeutic anticoagulation, we only recommend this approach when it has been less than 24 hours since onset of the AF episode. If the AF episode has lasted beyond 24 hours or it is unknown when the episode started, the risk of formation of intracardiac thrombus during AF and subsequent risk of stroke after a successful chemical cardioversion from a Class IC drug would be prohibitively high.
Due to the use-dependent nature of Class IC antiarrhythmics (more effective with more sodium channel blockade at faster ventricular rates), there is a chance of slowing conduction throughout the heart to the point that atrial fibrillation can organize into rapid atrial flutter with 1:1 AV conduction, leading to an aberrant wide complex tachycardia. For this reason, we recommend that the patient receive a beta-blocker or calcium channel blocker at least 30 minutes prior to administration of flecainide or propafenone.
Some practitioners recommend that if the patient is not already on anticoagulation, that they initiate anticoagulation at the time of beta-blocker or calcium channel blocker administration to reduce the risk of intracardiac thrombus formation if the patient does not convert to sinus rhythm within 24-48 hours.
Some practitioners recommend that the first attempt at “pill in the pocket” dosing be performed in the emergency department so that safety and efficacy can be monitored.
If patients report a progressively increasing need for “pill in the pocket” use or there is a suggestion of increasing burden of AF episodes, I recommend consultation with the patient’s cardiologist or electrophysiologist to discuss alternative options for rhythm control of symptomatic atrial fibrillation. Potential options at that time could include initiation of maintenance antiarrhythmic drug therapy versus invasive management with catheter ablation of atrial fibrillation.
When used in the right patient, a “pill in the pocket” approach can be a very effective strategy for rhythm control of infrequent symptomatic paroxysmal atrial fibrillation. Appropriate patient factors to consider prior to recommending this approach are nicely highlighted in the post above. “Pill in the pocket” management for AF can resolve patient symptoms, improve patient’s quality of life, and reduce unnecessary emergency room visits and subsequent hospitalizations.
References
Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014 Feb 25; 129(8):837-47.
Miyasaka Y, Barnes M, Gersh B, et al. Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation. 2006 Jul 11;114(2):119-25.
Kaustubha Patil, MD
Clinical Cardiac Electrophysiology
Bluhm Cardiovascular Institute Northwestern Medicine
Assistant Professor of Medicine
Northwestern University Feinberg School of Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Feiger, D. Andereck, J. (2020, Nov 2). A “Pill-in-the-Pocket” approach to paroxysmal atrial fibrillation. [NUEM Blog. Expert Commentary by Patil, K]. Retrieved from http://www.nuemblog.com/blog/pill-in-pocket.
Other Posts You May Enjoy
References
Adams, James, et al. “Tachydysrhythmias.” Emergency Medicine: Clinical Essentials, Elsevier Health Sciences, 2013, pp. 497–513.
Alboni, Paolo, et al. “Outpatient Treatment of Recent-Onset Atrial Fibrillation with the ‘Pill-in-the-Pocket’ Approach.” New England Journal of Medicine, vol. 351, no. 23, 2004, pp. 2384–2391., doi:10.1056/nejmoa041233.
Aliot, E., et al. “Twenty-Five Years in the Making: Flecainide Is Safe and Effective for the Management of Atrial Fibrillation.” Europace, vol. 13, no. 2, 2010, pp. 161–173., doi:10.1093/europace/euq382.
Besser, Kiera Von, and Angela M. Mills. “Is Discharge to Home After Emergency Department Cardioversion Safe for the Treatment of Recent-Onset Atrial Fibrillation?” Annals of Emergency Medicine, vol. 58, no. 6, 2011, pp. 517–520., doi:10.1016/j.annemergmed.2011.06.014.
Dan, Gheorghe-Andrei, et al. “Antiarrhythmic Drugs–Clinical Use and Clinical Decision Making: a Consensus Document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, Endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP).” EP Europace, vol. 20, no. 5, 2018, doi:10.1093/europace/eux373.
Dukes, I.d., and E.m Vaughan Williams. “The Multiple Modes of Action of Propafenone.” European Heart Journal, vol. 5, no. 2, 1984, pp. 115–125., doi:10.1093/oxfordjournals.eurheartj.a061621.
January, Craig T., et al. “2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation.” Journal of the American College of Cardiology, vol. 64, no. 21, 2014, doi:10.1016/j.jacc.2014.03.022.
Nuotio, Ilpo, et al. “Time to Cardioversion for Acute Atrial Fibrillation and Thromboembolic Complications.” Jama, vol. 312, no. 6, 2014, p. 647., doi:10.1001/jama.2014.3824.
“Pill-in-a-Pocket Dosing Safely Converts Breakthrough Atrial Fib.” Family Practice News, vol. 35, no. 18, 2005, p. 20., doi:10.1016/s0300-7073(05)71733-3.
Sacchetti, Alfred, et al. “Impact of Emergency Department Management of Atrial Fibrillation on Hospital Charges.” Western Journal of Emergency Medicine, vol. 14, no. 1, 2013, pp. 55–57., doi:10.5811/westjem.2012.1.6893.
Sethi, Naqash J., et al. “Digoxin for Atrial Fibrillation and Atrial Flutter: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomised Clinical Trials.” Plos One, vol. 13, no. 3, 2018, doi:10.1371/journal.pone.0193924.
Stiell, Ian G., et al. “Association of the Ottawa Aggressive Protocol with Rapid Discharge of Emergency Department Patients with Recent-Onset Atrial Fibrillation or Flutter.” Cjem, vol. 12, no. 03, 2010, pp. 181–191., doi:10.1017/s1481803500012227.
Wang, Z, et al. “Mechanism of Flecainide's Rate-Dependent Actions on Action Potential Duration in Canine Atrial Tissue.” American Society for Pharmacology and Experimental Therapeutics, vol. 267, no. 2, 1 Nov. 1993, pp. 575–581.
Yao, R., et al. “Real-World Safety And Efficacy Of A ‘Pill-In-The-Pocket' Approach For The Management Of Paroxysmal Atrial Fibrillation.” Canadian Journal of Cardiology, vol. 33, no. 10, 2017, doi:10.1016/j.cjca.2017.07.371.
Canadian Syncope
Written by: Jonathan Hung, MD (NUEM ‘21) Edited by: Jon Anderek (NUEM ‘19) Expert Commentary by: Andrew Moore, MD, MS
Introduction
Syncope is defined as a brief loss of consciousness that is self-limited. [1] It is a commonly seen chief complaint in the emergency department (ED), consisting of up to 3% of ED visits. [2] There are both benign causes of syncope such as vasovagal syncope and more serious causes such as arrhythmias. By the time these patients present to the ED, they are often asymptomatic and hemodynamically stable. Part of the ED workup and disposition includes risk stratification of these patients that can vary by provider and hospital system. [3] For those who present with high-risk features, ED physicians often recommend admission to the hospital for telemetry monitoring and expedited evaluation with echocardiography. [4] Multiple decision rules, most notably the San Francisco Syncope Rule (SFSR), have been developed to identify syncope patients at risk for poor outcomes. The SFSR takes into account predictors such as a history of heart failure, an abnormal electrocardiogram (ECG), and hypotension to determine 7-day negative outcomes for patients presenting to the ED with syncope. [5] Another study called the Osservatorio Epidemiologico sulla Sincope nel Lazio (OESIL) includes age over 65 and syncope without prodrome in addition to a history of cardiovascular disease as part of their decision-making tool. [6] Lastly, the Risk Stratification of Syncope in the Emergency Department (ROSE) also takes lab results such as brain natriuretic peptide and hemoglobin into account. [7] Despite the numerous studies examining risk stratification in syncope, each one has limitations and ultimately lack adequate sensitivity and specificity for widespread clinical adoption. A new study published in Academic Emergency Medicine is one of the largest studies to develop a risk tool that identifies adult syncope patients at 30-day risk for serious adverse outcomes defined as a serious arrhythmia, need for intervention to correct arrhythmia, or death. [8]
Study
Thiruganasambandamoorthy V, Stiell IG, Sivilotti MLA, et al. Predicting Short-term Risk of Arrhythmia among Patients With Syncope: The Canadian Syncope Arrhythmia Risk Score. Baumann BM, ed. Acad Emerg Med. 2017;24(11):1315-1326.
Study Design
Multi-center, prospective, observational cohort study.
This was a derivation study used to define the parameters of the risk score.
Population
Inclusion criteria:
Syncope patients presenting within 24 hours of the event
Adults age ≥16
Exclusion criteria:
Prolonged loss of consciousness
Change in mental status from baseline
Witnessed seizure
Head trauma or other trauma requiring admission
Unable to provide history due to alcohol intoxication, illicit drug use or language barrier
Obvious arrhythmia or nonarrhythmic serious condition on presentation
Intervention protocol
ED physicians and emergency medicine residents were trained to assess standardized variables at the initial ED visit including time and date of syncope, event characteristics, personal and family history of cardiovascular disease, and final ED diagnosis. Other variables were obtained through chart review and included age, sex, vital signs, laboratory results and ECG variables. All ECGs were reviewed by a cardiologist, and abnormal variables were reviewed by a second cardiologist. Physician gestalt for dangerous etiology was also recorded for each patient. Multivariable logistic regression was used for the analysis.
Outcome Measures
Composite of death, arrhythmia, or procedural interventions to treat arrhythmias within 30 days of ED disposition
Results
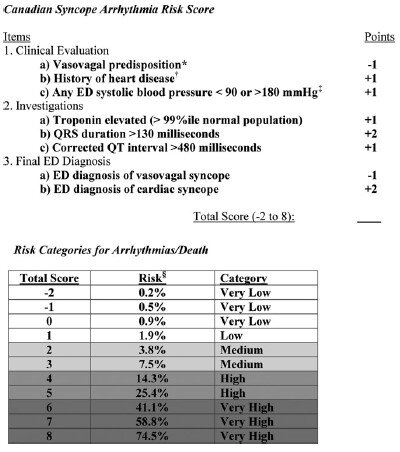
5,010 patients were enrolled in the study with 106 (2.1%) patients suffering arrhythmia or death within 30 days of ED presentation. Forty-five of the 106 patients suffered their adverse event outside of the hospital. The mean age of the study population was 53.4 (SD 23.0 years) and 54.8% were females. A total of 8 variables were included in the final model:
Vasovagal predisposition
History of heart disease (CAD, atrial fibrillation/flutter, CHF, valvular abnormalities)
Systolic blood pressure <90 or >180 mm Hg at any point
Troponin elevation
QRS duration >130 msec
QTc interval > 480 msec
ED diagnosis of cardiac syncope
ED diagnosis of vasovagal syncope
The Canadian Syncope Arrhythmia Risk Score had a sensitivity of 97.1% and specificity of 53.4% at a threshold score of 0 based on the study’s internal validation.
Interpretation
This study is the largest, multicenter study assessing predictors of short-term outcomes following initial ED presentation of syncope. The results are similar to previous studies that examined long-term outcomes. One interesting difference is that in prior studies, advanced age was a risk factor in arrhythmia or death, however it did not make the final model in this study. The strengths of this prospective study include the large patient population and that only 6.5% were lost to follow up. Furthermore, developing a simplified risk tool similar to the HEART score for chest pain, it can be easily utilized in the ED to help aid in decision making. Some limitations are that a large portion (54%) of patients did not have a troponin level measured and the study notes that these were usually younger patients with less comorbidities.
In practice, it may be difficult to use this tool if there is provider variation for when cardiac syncope is suspected and when a troponin level is measured. Whether or not the provider diagnoses vasovagal syncope or cardiac syncope is subjective as well, though may serve as a surrogate for “physician gestalt.” These results are helpful in risk stratifying syncope patients especially in regard to short-term outcomes, however this disease process is complex and cannot be oversimplified. Overall, this decision tool at the very least allows ED providers to have a shared decision-making conversation with more robust data to support the various options.
Take Home Points
The Canadian Syncope Arrhythmia Risk Score is a large, multicenter trial evaluating serious 30-day outcomes following an ED presentation for syncope
Emergency medicine physicians may consider using this tool to guide their clinical-decision making for syncope patients by offering risk percentages for 30-day adverse events
At the time this was written a validation study was underway
Expert Commentary
The management and disposition of syncope has been a conundrum for emergency physicians for decades. In fact, the last 20 years of syncope research have focused on development of a risk stratification score for the ED management of syncope. With the recent external validation of the Canadian Syncope Risk Stratification Score [9] (CSRSS) and the recent publication of the FAINT Score [10] for syncope in older adults, we now have two prospectively derived studies to support risk stratification of the syncope patient. The external validation of the CSRSS showed good sensitivity for low risk patients with a sensitivity of 97.8%. None of the very low risk or low risk patients in the external validation died or suffered cardiac arrhythmia in 30 days. Based on this if your patient is very low risk or low risk you can safely discharge the patient home with primary care follow up.
In my practice, the CSRSS serves as an adjunct to clinician judgement. Using a risk stratification score is often the impetus for a shared decision-making discussion regarding risk and safe disposition. The results of the external validation study further support clinical use of the CSRSS.
The FAINT score also shows promise for risk stratification in older patients with syncope and near syncope. This score has not been externally validated, but focuses on the older population that many emergency physicians reflexively admit for cardiac monitoring.
Regardless of which decision score you decide to use in personal practice, most of these patients with unexplained syncope can be safely admitted for a short observation stay. It is safe to say that we have entered a golden age of syncope decision rules.
Andrew Moore, MD, MS
Emergency Physician and Emergency Care Researcher
Department of Emergency Medicine
Carilion Clinic
How To Cite This Post:
[Peer-Reviewed, Web Publication] Hung, J, Anderek, J. (2020, Sept 14). Canadian Syncope. [NUEM Blog. Expert Commentary by Moore, A]. Retrieved from http://www.nuemblog.com/blog/canadian-syncope.
Other Posts You May Enjoy
References
Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. European heart journal 2018;39:1883-948.
Sun BC, Emond JA, Camargo CA, Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992-2000. Acad Emerg Med 2004;11:1029-34.
Probst MA, Kanzaria HK, Gbedemah M, Richardson LD, Sun BC. National trends in resource utilization associated with ED visits for syncope. The American journal of emergency medicine 2015;33:998-1001.
Cook OG, Mukarram MA, Rahman OM, et al. Reasons for Hospitalization Among Emergency Department Patients With Syncope. Acad Emerg Med 2016;23:1210-7.
Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Annals of emergency medicine 2004;43:224-32.
Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. European heart journal 2003;24:811-9.
Reed MJ, Newby DE, Coull AJ, Prescott RJ, Jacques KG, Gray AJ. The ROSE (risk stratification of syncope in the emergency department) study. J Am Coll Cardiol 2010;55:713-21.
Thiruganasambandamoorthy V, Kwong K, Wells GA, et al. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2016;188:E289-98.
Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, et al. Multicenter Emergency Department Validation of the Canadian Syncope Risk Score. JAMA internal medicine 2020;180:1-8.
Probst MA, Gibson T, Weiss RE, et al. Risk Stratification of Older Adults Who Present to the Emergency Department With Syncope: The FAINT Score. Annals of emergency medicine 2019.
D-dimer for Aortic Dissections and Acute Aortic Syndromes
Written by: Samantha Stark, MD (NUEM ‘20) Edited by: Jesus Trevino (NUEM ‘19) Expert Commentary by: Keith Hemmert, MD
The diagnosis of aortic dissections and other acute aortic syndromes (AAS) has long plagued the emergency physician due to the non-specific nature of their presenting symptoms and the potentially catastrophic consequences of a missed diagnosis. While there is increasing interest and a growing body of evidence regarding the use of D-dimer in diagnosis, guidance on how to use the D-dimer and comfort in doing so are lacking. In the ADvISED study (Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes), the safety and efficiency of integrating a pretest probability assessment with D-dimer testing is evaluated.
This was a multicenter prospective observational study that involved 6 hospitals in 4 different countries from 2014-2016, including 1850 patients. They observed the failure rate and efficiency of a diagnostic strategy for ruling out AAS that involved determining pretest probability and combining this with a D-dimer test. The tool used for assessing pretest probability was the aortic dissection detection risk score (ADD-RS, see below) and the D-dimer was considered negative if <500 ng/mL. As above, primary and secondary outcomes were the failure rate and efficiency of this strategy, respectively.
*For each risk category, one point is assigned if one or more risk factors is present. The ADD-RS can therefore vary from 0-3.
For the purposes of this study, it is appropriately assumed that anyone with ADD-RS>1 would need conclusive testing (CTA, TEE, or MRA) to evaluate for AAS regardless of D-dimer level. Therefore, the investigators looked primarily at the integration of negative D-dimer (DD-) testing with ADD-RS=0 or ADD-RS<1 as a possible rule out strategy for AAS. They found that among ADD-RS=0/DD- patients, the failure rate was 0.3% (1/294 patients, 95% CI, 0.1-1.9) and the efficiency in ruling out AAS was 15.9% (1/6 patients, 95% CI, 14.3-17.6); efficiency was computed as the number of patients with negative D-dimer within a risk category divided by the number of enrolled patients). Among ADD-RS<1/DD- patients, the failure rate was also 0.3% (3/924 patients, 95% CI, 0.1-1) and the efficiency was 49.9% (1/2 patients, 95% CI, 47.7-52.2). Of note, as mentioned above, in patients with ADD-RS>1/DD-, the failure rate was 4.4%, corresponding to 1 missed case for every 22 patients, an unacceptable failure rate for a potentially lethal condition.
While the study’s statistical methods were thorough and sound, and the results quite compelling, there were some issues with the study. Perhaps the most significant of these was that about half of the patients involved in the study did not undergo conclusive diagnosis for AAS (CTA, TEE, MRA, surgery, or autopsy), and their “case adjudication” was based on 2-week follow up data. Of the cases where presence or absence of AAS was determined based on follow up data alone, 13% were determined to have AAS, while 87% were determined to have other explanations for their symptoms. The authors make the arguably legitimate, but unvalidated, assumption that patients with undiagnosed symptomatic AAS would experience some significant clinical event in the 2-week time period from presentation to follow-up. This is further supported by the fact that they did identify 7 cases of AAS during said follow-up period. However, they state that, “nonetheless, we cannot exclude with certainty that in 731 study patients with ADD-RS<1/DD- and a negative 14-day follow-up, few cases of AAS with mild or atypical manifestations might have been missed.”
The authors also point out that there is no established acceptable failure rate of a rule out strategy for AAS. They extrapolate based on prior studies that showed a) the threshold clinical probability of AAS above which the benefits of testing outweigh the risks was 3% and b) similar strategies for PE rule out have been considered acceptable if the upper limit of the 95% CI around the failure rate was <3%, to suggest that the failure rate of 0.3% for both the ADD-RS=0/DD- and the ADD<1/DD- strategies (with upper limits of the 95% CI 1.9% and 1% respectively) could be considered acceptable. That suggestion is intriguing when there is data showing the misdiagnosis rate of AAS reaching as high as 40% and that a mere 2.7% of CTAs obtained to evaluate for AAS yield a positive result.
They conclude that integration of ADD-RS=0 or ADD-RS<1 with negative D-dimer testing may be considered to standardize the diagnostic rule out of AAS, and that expert evaluation and debate are needed to determine whether the outlined strategies are safe and efficient to be recommended and implemented in clinical practice. Below is a flowchart from the paper summarizing their proposed diagnostic approach.
Expert Commentary
Thank you for this thoughtful review of the ADvISED trial. Acute Aortic Syndromes (AAS), including aortic dissection, intramural hematoma, ulcer, and rupture, are a challenging set of pathologies for the Emergency Physician. As Drs. Stark and Trevino note in this excellent post, the clinical presentations are often vague, and the mortality rate is high if the diagnosis is missed. The approach to testing for AAS has historically not been based on any validated risk score, but rather on clinical gestalt. As a result of these factors the yield on diagnostic testing for AAS is low, raising concerns about resource use and radiation exposure. Hence, a validated approach to risk stratification, with an acceptably low miss rate, would be a great aid to the Emergency Physician.
It is always important to bear in mind the current standard of care for AAS. The American College of Emergency Medicine currently does not recommend the use of a clinical decision rule alone to rule out AAS, nor does it recommend the use of d dimer alone to rule out AAS. CTA, MRA and TEE are the recommended modalities to diagnose of rule out AAS. [1] While this study and others may ultimately be a factor in changing ACEP clinical policies, Emergency Medicine house staff should be mindful of the current standard of care when evaluating new diagnostic or risk stratification strategies.
As both the authors and Drs. Stark and Trevino point out, roughly half of the patients in the study did not have gold standard testing for AAS, whether by CTA, MRA, TEE, or autopsy; they were simply assumed to be negative for AAS. While it is difficult to find reliable data on the true mortality rate of untreated AAS, we can use mortality for acute aortic dissections as a proxy. The cumulative 14-day mortality for treated acute aortic dissections (including type A and type B, and both medical management and surgical management) approaches 50%. [2] One can presume that the mortality rate for untreated disease would be even higher. While this does lend a modicum of logic to the authors’ approach (if they aren’t dead at 14 days, they probably don’t have AAS), it is far from scientific, and fails to meet the bar for the level of evidence required to forego conclusive diagnostic testing for such a lethal pathology.
The highly lethal nature of AAS also raises the question of the acceptable miss rate for a risk stratification tool such as the one proposed in the ADvISED study. As mentioned, a tool like this will inevitably draw comparisons to the PERC rule, which has a failure rate of <2%. However, AAS is a substantially more lethal disease than PE; the 30-day mortality rate of PE is 4%, and the 1-year mortality rate is 13%. [3] While an apples to apples comparison of the mortality rates of PE and AAS is challenging, the aforementioned 14 day mortality rate for treated AAS (nearly 50% at 14 days) provides a stark contrast. Additionally, AAS encompasses a variety of discrete pathologies, some of which are extraordinarily lethal (e.g., the mortality of an ascending aortic dissection is 1% to 2% per hour after symptom onset). [2] To use another proxy, the in-hospital mortality rate for type A dissections is 22%, and for type B 13%. [4] These numbers, of course, discount the (presumably not insignificant) number of patients who die before completing transfer to a quaternary care facility.
All of this is to say that AAS is a much more lethal set of pathologies than PE, and therefore the acceptable failure rate for a risk stratification strategy must be correspondingly lower. The authors report a failure rate for the most conservative option (ADD-RS=0/DD-) of 0.3% - but this must be interpreted in light of the lack of conclusive diagnostic imaging in roughly half of the patients enrolled in the study. Lastly, a comparison of this strategy to clinical gestalt would enable us to evaluate the superiority of this approach to the current one; this is a ripe area for future investigation. In summary, the approach to AAS proposed in the ADvISED study is not ready for widespread implementation, although it is a promising step toward a usable risk stratification strategy.
References
1. ACEP. ACEP Clinical Policy on Thoracic Aortic Dissection
2. Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112(24):3802-3813. doi:10.1161/CIRCULATIONAHA.105.534198
3. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular Trends in Incidence and Mortality of Acute Venous Thromboembolism: The AB-VTE Population-Based Study. Am J Med. 2016;129(8):879.e19-879.e25. doi:10.1016/j.amjmed.2016.01.041
4. Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the international registry of acute aortic dissection: A 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846-1860. doi:10.1161/CIRCULATIONAHA.117.031264
Keith Hemmert, MD
Assistant Professor of Emergency Medicine
Hospital of the University of Pennsylvania
Other Posts You May Enjoy
References
1. Nazerian P, Mueller C, Matos Soeiro A, Leidel B, Savadeo SAT, Giacino F, Vanni S, Grimm K, Oliveira MT, Pivetta E, Lupia E, Grifoni S, Morello F. Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes: The ADvISED Prospective Multicenter Study. Circulation. 2018;137:250-258.
2. Righini M et. al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117-1124.
3. Perrier A et. al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
4. Van Belle A et. al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172-179.
5. Sarasin FP et. al. Detecting acute thoracic aortic dissection in the emergency department: time constraints and choice of the optimal diagnostic test. Ann Emerg Med. 1996;28:278-288.
6. Hansen MS et. al. Frequency of an inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99:852-856.
7. Kurabayashi M et. al. Factors leading to failure to diagnose acute aortic dissection in the emergency room. J Cardiol. 2011;58:287-293.
8. Zhan S et. al. Misdiagnosis of aortic dissection: experience of 361 patients. J Clin Hypertens (Greenwich). 2012;14:256-260.
9. Lovy AJ et. al. Preliminary development of a clinical decision rule for acute aortic syndromes. Am J Emerg Med. 2013;31:1546-1550.
A Closer Look at the HEART Score
Written by: Abiye Ibiebele, MD (PGY-3) Edited by: Kumar Gandhi, MD (PGY-4) Expert commentary by: D. Mark Courtney, MD, MCSI
Chest pain accounts for nearly six million annual visits to the Emergency Departments across the United States and accounts for over ten billion in healthcare dollars, and thus the appropriate management of chest pain is part of the daily reality of Emergency Physicians. From one’s first EM rotation in medical school, one learns to always rule out the dangerous causes of chest pain first, of which acute coronary syndrome (ACS) is often first and foremost. A STEMI is called from the field or quickly on arrival to the emergency department and the patient is quickly evaluated by cardiology either in the emergency department or on the way to the cardiac catheterization lab. However, the diagnostic and management challenge remains for those patients that may have ACS without initial EKG changes or an elevation in troponin. To help us, clinical decision rules have been developed to help us identify which patients who present with chest pain we can safely rule out life-threatening ACS in and help risk stratify patients into different risk categories [1]. What follows is a breakdown of the components of the HEART score, a review of how to appropriately assign points and a brief review of the original study and subsequent research since then.
HEART Score
Backus et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9(3):164-169.
History
This is the most subjective area of scoring in the HEART score and one of possible contention between different health care providers.
The original study broke down historical elements as specific for ACS and nonspecific for ACS as judged by the clinical experience of practiced providers.1 A 0 score, was given for a completely nonspecific history and a 2 score was given for a primarily specific history. For a mixture of nonspecific and specific elements, a 1 score was given.
The original researchers used clinical gestalt and took into account historical elements such as pattern of pain, onset, duration, relation to exercise, localization, concomitant symptoms and reaction to sublingual nitrates. While this was based on clinical judgment, the historical elements were somewhat based on a prior clinical review which listed specific elements as follows: [3]
Concerning history (read: specific for ACS)
Chest pain radiating to one or both arms
Pressure like pain with associated nausea, vomiting, or diaphoresis
Exertional chest pain
Response of chest pain to nitroglycerin
Chest pain similar to prior MI
Non-concerning history for ACS (read: nonspecific for ACS)
Pleuritic or positional chest pain
Chest pain reproducible with palpation
Stabbing quality of pain
Pain localized to an area on chest smaller than a coin
It is important to note is that for assigning a history score, developers did not take into account risk factors or EKG findings. These are accounted for elsewhere in the HEART score.
EKG
Two points are assigned for ST elevations or depressions, in the absence of a bundle branch block, LVH or use of digoxin [1].
One point is assigned for repolarization abnormalities (new or old) without ST depression. A person can also receive a score of 1 for a bundle branch block or left ventricular hypertrophy [1].
Zero points is assigned for a normal EKG [1].
Age
This component of the HEART score is the most straightforward with scoring as defined in the chart above.
Risk Factors
As in the above chart, having no risk factors results in a score of zero points. Having 1-2 risk factors yields a score of 1. Important thing to note is about having at least 3 risk factors OR a “history of atherosclerotic disease” results in a score of 2 points. [1]
What does “history of atherosclerotic disease” mean? [1]
History of revascularization (PCI or CABG)
History of myocardial infarction
History of ischemic stroke
History of peripheral arterial disease
Thus, a patient with a history of any of the above disease should automatically get a 2 for this section of the HEART score.
What were included as risk factors for study purposes? [1,2]
Hyperlipidemia
Hypertension
Diabetes Mellitus
Cigarette smoking (has to have last smoked within 90 days)
Family history of coronary artery disease (doesn’t matter if family member was over/under 50 years of age)
Obesity (defined as BMI over 30)
Troponin
Also, a straightforward component of the HEART score with scoring as above.
Some notes regarding scoring:
Original study and validation studies did not use high sensitivity troponin when calculating the HEART score [1,4]
Follow up studies have used high sensitivity troponins in what is referred to as a “modified HEART score”. Scoring is similar to conventional troponin testing as in above chart. [5,6]
As those of you who have use the HEART score know, you calculate all the points and if a patient has a score between 0-3, they are considered low risk can be discharged home safely. A score between 4-6 is considered moderate risk and should be admitted for further observation and workup. A score of 7-10 is considered high risk and is recommended to have an early invasive intervention.
Now that we have covered the components of the HEART score, as previously mentioned, below is a brief review of the original study. However, before that, two caveats to using the HEART score:
For original study and following validation studies, patients who presented with only dyspnea or palpitations without associated chest pain were excluded [1,4]
HEART score has been shown to be helpful in distinguishing risk even when looking within special populations (diabetics, elderly, and females) [1,2,4,5]
Original Study
Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191-196.
Study design
Retrospective, single center study at a 265 bed community hospital in the Netherlands
Inclusion Criteria
Patients included were any patient admtted to the ER due to chest pain irrespective of age, prehospital assumptoms and previous medical treatments
Patients with STEMI were excluded
Methods
Patient’s charts were reviewed and HEART score was calculated as above. Endpoints as described below were identified and differences between groups were statistically analyzed.
Endpoints
Acute MI, revascularization, death and composite endpoint of all three.
Demographics
Total of 122 patients, mean age 61 years old, 60% male, race not specifically measured but overall hospital population >95% White/ Caucassion.
Results
All patients:
24% of all patients reached one or more of above endpoints
Average HEART score for all patients that did not reach an endpoint: 3.71 +/- 1.83
Average HEART score for all patients that did meet an endpoint: 6.51 +/- 1.84
Significant difference p < 0.0001
Heart Score Groups:
For patients with HEART score 0-3, 2.5% reached an endpoint
For patients with HEART score 4-6, 20.3% reached an endpoint
For patients with HEART score 7-10, 73% reached an endpoint
What to do with low risk patients?
Based on the heart score original study, for low risk patients (HEART score 0-3) the risk of a major adverse cardiac event (MACE) was 2.5%. The original suggestion from the authors of this study is that these patients can be immediately discharged.
Further validation studies have shown the risk of MACE within 45 days to be 1.9% and of all-cause mortality to be 0.05%.
There has also been development of the HEART score pathway, which adds a 3-hour serial troponin to low risk patients. In the initial study, 40 patients were discharged from the ED after two negative troponins and none of those patients had a MACE within 30 days.7
A 2017 systematic review of 9 studies and 11,217 patients revealed that 1.6% of the low risk patients would have a MACE at 6 weeks8. This study derived the sensitivity of the HEART score to be 96.7%. Another systematic review is ongoing to validate these findings and to further delineate the prognostication value of the HEART score9
Ultimately, having a discussion with these patients about what low risk means and coming to a shared decision can be a useful tool but in a patient with good outpatient follow-up, it is reasonable to discharge the patient without further cardiac testing,
Expert Commentary
What clinicians (and patients) care about is post test probability. This is a function of two things, what the pretest probability is and how good your testing tools are. The HEART score, the modified HEART score and the HEART Score Pathway as described above are all ways to try to formalize the process of pretest probability estimation. It is important to realize that the standard ED evaluation is suited to identify STEMI and NSTEMI. However part of the definition of MACE is angina and that is the entire point of stress testing….to discover treatable intermittent cardiac ischemia that may not show up on initial or subsequent ECGs or troponin testing. The HEART score approach has rapidly become the most widely used approach in the US to try to determine who needs a stress test during the index admission vs. who may be able to safely be discharged for follow-up and potential stress testing as an outpatient.
Despite popularity, there are challenges with any HEART score approach. These are as follows. 1) Clinicians like to talk about the HEART score but often don’t document it or do so in a template dot-phrase manner. All decision support pretest probability scores are only as good as the degree of accuracy of the data applied and documented. At times documentation is simply “HEART SCORE low” without the granular details to support this in the record. 2) There is potential for inter-observer disagreement. In work presented in abstract at the 2018 SAEM annual meeting Dr. Steven Ignell reported that residents were less likely to classify patients as low risk than attendings, and that formal application of the HEART score did not result in a larger proportion of low risk classification than by simple gestalt alone (do you think this patient is at 2% risk of 6 week MACE?). However in this work the agreement was better using the HEART score with a weighted Kappa of 0.62 vs 0.29 for gestalt along. So it seems that the HEART score likely identifies a roughly similar cohort of patients but does so with more standardization. The biggest future questions are to what degree in the US a HEART score approach will be optimally integrated with hsTroponin testing, who needs serial hsTroponin testing and who does not, and does timing of pain onset matter? Ongoing and future trials may bring insights into these important process questions.
D. Mark Courtney, MD, MSCI
Associate Professor
Department of Emergency Medicine
Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Ibiebele A, Gandhi K. (2019, Dec 9). A Closer Look at the HEART Score. [NUEM Blog. Expert Commentary by Courtney DM]. Retrieved from http://www.nuemblog.com/blog/HEART-score.
References
Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191-196.
Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9(3):164-169.
Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294(20):2623-2629.
Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158.
Ma CP, Wang X, Wang QS, Liu XL, He XN, Nie SP. A modified HEART risk score in chest pain patients with suspected non-ST-segment elevation acute coronary syndrome. Journal of geriatric cardiology : JGC. 2016;13(1):64-69.
Santi L, Farina G, Gramenzi A, et al. The HEART score with high-sensitive troponin T at presentation: ruling out patients with chest pain in the emergency room. Intern Emerg Med. 2017;12(3):357-364.
Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203.
Van Den Berg P, Body R. The HEART score for early rule out of acute coronary syndromes in the emergency department: a systematic review and meta-analysis. European heart journal Acute cardiovascular care. 2018;7(2):111-119.
Byrne C, Toarta C, Backus B, Holt T. The HEART score in predicting major adverse cardiac events in patients presenting to the emergency department with possible acute coronary syndrome: protocol for a systematic review and meta-analysis. Systematic reviews. 2018;7(1):148.
Other Posts You Might Enjoy…
Don't Forget aVL!
Written by: Laurie Aluce, MD (NUEM PGY-3) Edited by: Kimberly Iwaki MD (NUEM Alum ‘18 ) Expert commentary by: Daniel Schimmel, MD
The Case:
Patient: 57 year old male
Chief Complaint: Chest Pain
PMH: Hypertension
Pain: Mid-sternal, non-radiating, 6/10, constant pressure
Onset: 45 minutes ago while watching TV
Associated symptoms: No diaphoresis, light-headedness, nausea, vomiting, or back pain
Vitals: T 98.8, HR 70, BP 110/68, RR 14, Sat 100% on room air
Physical Exam:
General: Alert, no apparent distress, sitting comfortably in cart
Cardiac: Regular rate and rhythm, no murmurs/rubs/gallops
Pulmonary: Clear to auscultation bilaterally, no crackles/wheezes
Extremities: Warm and well perfused, 2+ distal pulses, cap refill < 2s
Labs: In process
Triage EKG:
Image from Stephen Smith, MD. http://hqmeded-ecg.blogspot.com/2011/02/inferior-hyperacute-t-waves-clue-is-t.html
What abnormalities do you see in this EKG?
Prolonged PR interval
Slightly widened QRS
Hyperacute T waves in II, III, aVF
T wave inversion (TWI) in aVL
< 1mm ST depression in I, avL
What are you concerned about? What elements of this EKG raise your concern?
Inferior myocardial infarction (MI) due to hyperacute T waves in II, III, aVF
Inferior MI or significant mid left anterior descending (LAD) lesion due to TWI in aVL [3]
How will you differentiate an inferior MI from a chronic mid LAD lesion?
Clinical picture and a repeat EKG. If the TWI is due to a chronic mid LAD lesion, it should not change on serial EKGs. If the TWI is due to an inferior MI, you may see it evolve to ST depression in aVL and/or ST elevation in II, III, aVF. [3]
You obtain a repeat EKG with one right-sided lead, V4R:
Image from Edward Burns, MD. https://lifeinthefastlane.com/ecg-library/basics/inferior-stemi/
What abnormalities do you see in the repeat EKG?
Hyperacute T waves and ST elevation in II, III, aVF
ST depression and TWI in aVL
ST depression in V2
<1mm ST depression in I
<1mm ST elevation in V4R
What are you concerned about? What elements of this EKG raise your concern?
Concern for inferior MI
ST elevation in II, III, aVF
ST depression and T wave inversion in aVL
EKG sensitivity for inferior MI increases from 60% to 77% when criteria includes ≥ 1mm reciprocal ST depression in aVL in addition to ≥ 1mm ST elevation in II, III, aVF [1]
Concern for right ventricle (RV) involvement
25% – 53% of inferior MIs have RV involvement [5]
ST elevation in III > II
Isoelectric ST segment in V1 with ST depression in V2
ST depression and T wave inversion in aVL
>1mm ST depression in aVL has sensitivity of 87% and positive predictive value of 90% for RV involvement with acute inferior MI [5]
Other findings suggestive of RV involvement not pictured in this EKG
ST elevation in V2 > 50% the magnitude of ST depression in aVF [4]
Right bundle branch block, 2nd and 3rd degree AV block
ST elevation in V1
How can your further evaluate for RV involvement?
You ask for a right-sided EKG to check for ST elevation in V3R-V6R, but your EKG tech doesn’t remember where to place the leads. The tech looks to you for help. Where do you put the leads?
Full Right-Sided EKG Standard EKG with V4R
Images from Tom Bouthillet. http://www.ems12lead.com/2008/10/17/12-lead-ecg-lead-placement-diagrams/
If you don't have the time to perform a full right-sided EKG, you can perform a standard EKG but switch V4 to V4R as shown with the prior EKG. V4R is considered the best single lead for diagnosing a right ventricular MI.[2] ST elevation ≥ 1 mm in V4R has a sensitivity of 88% and a specificity of 78% for RV infarction. [6]
EKG with V4R-V6R:
Image from Ary Goldberger, MD. UpToDate: Electrocardiogram in the diagnosis of myocardial ischemia and infarction
What abnormality do you see in the right-sided leads?
ST elevation in V4R-V6R
What is your diagnosis?
Inferior MI with RV infarction
Take Home Points:
Don’t forget aVL. T wave inversion and ST depression in aVL can be a marker of serious cardiac pathology:
Significant mid-LAD lesion
Evolving inferior MI and possible RV involvement
If you are concerned for inferior ischemia, obtain a right-sided EKG to evaluate for right ventricular involvement
Expert Commentary
This is an excellent review of aVL, an important lead for diagnosis and prognosis. Because it can be a modifier, or is used in conjunction with other criteria for diagnosis, it often gets overlooked.
In the acute setting, aVL can be used to help identify ST elevation myocardial infarction (STEMI) in various locations. In addition to the aforementioned use in identifying RV involvement in inferior STEMI and mid LAD lesions, aVL is considered a high lateral lead which is fed by diagonal vessels. As such, acute blockages that affect the proximal LAD with anterior STEMI in V3, V4, anteroseptal involvement in V1, V2 and may also have high lateral involvement in aVL. While I am always watching for shock and unstable arrhythmias taking patients to the cardiac catheterization lab, if I see this distribution with an anterior STEMI, I am even more concerned due to the amount of myocardium subtended by the lesion. I often preemptively get access for cardiac mechanical support in case cardiogenic shock develops. In this way, the prognostic information from aVL may change practice in the cardiac catheterization lab.
Not to be overlooked, leads I and aVL may be the only leads with ST elevation in an isolated high lateral STEMI in a diagonal vessel. Diagonal 1 or diagonal 2 may be large vessels and be the only vessel with acute thrombosis. aVL is the reciprocal lead to the inferior leads in inferior STEMI. Similarly, lead III may move reciprocally, showing ST segment depression in the high lateral STEMI with elevation in aVL.
As nice as it can be to have aVL to prognosticate inferior STEMI, prognosticate anterior STEMI and identify isolated high lateral STEMI, aVL ST segments and T waves can be abnormal for non-acute reasons leading to a lack of specificity. The most common reason for an abnormal repolarization in aVL is strain in the setting of LVH. So how do we differentiate acute reciprocal changes from strain pattern?
A frequently overlooked bit of information on the EKG is the QRS-T angle. Luckily this is reported for us on the EKG at the top. In the setting of T wave inversion, if the angle is greater than 100 degrees, this is more consistent with strain. Furthermore, if the ST segment leading to the T wave is downwardly concave (think downward parabolic curve) with asymmetry of the T wave, this also is more consistent with strain. If we look at the presented case’s EKGs, we can dissect them a bit further. In the triage EKG, there isn’t ST segment depression at all. In fact there is elevation with a T wave inversion. This is concerning. The ST segment may be isoelectric with an inverted T wave, but there should not be ST elevation with a T wave inverted in the opposite direction. This morphology is often used in Sgarbossa criteria to identify STEMI in left bundle branch block. So in addition to the hyperacute T waves of this triage EKG, aVL is very concerning. In the follow-up EKG, we can see dynamic changes that have occurred in the inferior leads solidifying the diagnosis. But aVL also suggests RV involvement. The ST segment is downsloping, depressed without concavity (more linearly pointing down to the right). To clinch the diagnosis of RV involvement, we also see the right sided leads with elevation.
It is definitely possible to have a patient with LVH/strain findings and also have an acute inferior STEMI. As demonstrated successfully in this case, the statement about serial EKGs is a critical one. Even in the setting of a normal EKG, a good story for myocardial infarction should prompt a serial EKG and often a posterior EKG to identify a supposedly electrocardiographically silent STEMI. Remember STEMI is an EKG diagnosis that is supposed to correlate with the physiologic condition of an acute 100% occlusion of a vessel. In the early period, there can be stuttering pain with opening and closing of the vessel and the EKG may not capture it in that moment or may miss the posterior STEMI if the posterior EKG is not obtained.
To sum up the importance of aVL:
Inferior STEMI: Improved diagnosis when aVL has a depressed ST segment with an inverted T wave
Inferior STEMI: Considered worse prognosis when aVL has a depressed ST segment with an inverted T wave as it suggests a more proximal RCA occlusion with RV involvement. ST elevation in V1 or right sided V4 may also be helpful in identifying proximal RCA occlusion with RV branch involvement.
Anterior STEMI: aVL STEMI confers a worse prognosis, suggesting more proximal LAD involvement.
Isolated high lateral STEMI: ST elevation in leads I and aVL may identify an acute 100% blockage in a diagonal vessel. Lead III may have reciprocal ST segment depression.
To differentiate strain from ischemic changes in aVL:
QRS-T angle: If greater than 100, may be consistent with strain.
ST segment slope: If concave, consider strain. If downsloping but the ST segment is more straight as it aims downward, consider an acute event.
T Wave inversion: If there is asymmetry to the inverted T wave, it may be more consistent with strain.
In summary, the EKG has so much data in it. Pattern recognition can be a wonderful tool for quick diagnosis of clear problems. But a methodical evaluation of the EKG can still be done quickly and identify what others may miss.
Dr. Daniel R. Schimmel
Assistant Professor of Medicine
Bluhm Cardiovascular Institute
Northwestern University
References:
1. Birnbaum Y, et al. ST segment depression in aVL: a sensitive marker for acute inferior myocardial infarction. Eur Heart J 1993;14(1):4-7.
2. Carley SD. Beyond the 12 lead: Review of the use of additional leads for the early electrocardiographic diagnosis of acute myocardial infarction. Emergency Medicine 2003;15: 143-54.
3. Hassen GW, et al. Lead aVL on electrocardiogram: emerging as important lead in early diagnosis of myocardial infarction. Am J Emerg Med 2014;32(7)785-88.
4. Somers MP, et al. Additional electrocardiographic leads in the ED chest pain patient: right ventricular and posterior leads. Am J Emerg Med 2003;21(7):563-73.
5. Turhan H, et al. Diagnostic value of aVL derivation for right ventricular involvement in patients with acute inferior myocardial infarction. Ann Noninvasive Electrocardiol 2003;8:185-88.
6. Zehender M, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328:981-88.
How to Cite this Post
[Peer-Reviewed, Web Publication] Aluce L, Iwaki K. (2019, Aug 12). Don’t Forget aVL. [NUEM Blog. Expert Commentary by Schimmel D]. Retrieved from http://www.nuemblog.com/blog/dont-forget-aVL/.
Other Posts You Might Enjoy
Lovenox in NSTEMI
Written by: Terese Whipple, MD (NUEM PGY-3) Edited by: Andrew Ketterer, MD (NUEM Alum ‘17) Expert commentary by: Michael Macias (NUEM Alum ‘17)
The Case
A 64 year old female with history of diabetes and hypertension is brought to your emergency department (ED) by EMS for “acid reflux.” She has new T wave inversions in leads II, III, and aVF with a troponin of 0.12. The patient is given aspirin, nitroglycerine, and ticagrelor, but before signing your heparin drip order, you ask yourself:
Would enoxaparin (LMWH) be a better option?
The Recommendation:
The AHA and ACC guidelines state, “In patients with NSTE-ACS, anticoagulation, in addition to antiplatelet therapy, is recommended for all patients irrespective of initial treatment strategy. Treatment options include:
Enoxaparin: 1 mg/kg subcutaneous every 12 hours, continued for the duration of hospitalization or until PCI is performed. An initial IV loading dose is 30 mg (Level of evidence A)
Unfractionated heparin (UFH) IV: initial loading dose 60 IU/kg (max 4000 IU) plus 12 IU/kg/h (max 1000 IU/h) adjusted per activated PTT in according to specific hospital protocol (Level of evidence B)”[2]
Bivalirudin/Fondaparinux- These are other options outside the scope of this post
The Evidence:
So why is the evidence for enoxaparin Level A and UFH level B? Multiple randomized controlled trials have examined this issue:
The ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events) trial was the first major RCT to demonstrate the efficacy of enoxaparin over UFH. Although it was conducted in the late 90s, before GIIb/IIIa inhibitors or early invasive intervention were commonplace, it demonstrated a statistically significant decrease in the risk of death, MI, or recurrent angina in those randomized to treatment with enoxaparin (16.6%) v. UFH (19.8%) (p=.019). Incidence of major bleeding was similar in both groups.
TIMI-II: Enoxaparin was found to be superior with no increase in hemorrhage. Outcomes measured were death, MI, and urgent revascularization at 8 and 43 days, respectively, for enoxaparin (12.4%, 17.3%) and UFH (14.5%, 19.7%) (P=.048).
SYNERGY: In patients undergoing early PCI, enoxaparin was not inferior to UFH in the treatment of NSTEMI. However, enoxaparin was associated with more major bleeding (3.7% v 2.5%, p=.028). Both bleeding and efficacy were potentially confounded by crossover from LMWH before randomization to UFH upon randomization and vice versa … more below.
A to Z: Studied the efficacy and safety of enoxaparin and tirofiban compared w/ UFH and tirofiban. Incidence of death, MI, refractory ischemia with enoxaparin (8.4%) v. UFH (9.4%) were similar with similar bleeding incidences. Enoxaparin was found to be not inferior to UFH.
A systematic review conducted in 2004 of approximately 22,000 patients extending across the evolution of ACS treatment from conservative management to early PCI, demonstrated that enoxaparin is more effective than UFH in preventing MI and death.
It may seem that as treatment of NSTEMI has evolved to include antiplatelet therapy and early invasive intervention, the superiority of enoxaparin has been negated. However, both the SYNERGY and A to Z trials were potentially confounded by the fact that a majority of patients received pre-randomization therapy. A subgroup analysis performed on those patients who received only enoxaparin in the SYNERGY trial and no pre-treatment anticoagulation demonstrated the superiority once again of enoxaparin over UFH in regards to the combined outcome of death and MI (13.3% vs. 15.9% , p= 0.004). The bleeding risk also seemed to be increased by pre-randomization therapy. The subgroup analysis showed no significant difference in major bleeding between UFH and enoxaparin when the patients received enoxaparin only (OR 1.04, CI 0.83-1.3).
If changing anticoagulation potentially increases bleeding risk, what about those destined for PCI?
The practices and procedures involved in PCI are beyond the scope of emergency medical practice, however the medications that we choose in the ED have downstream effects on patient care. Therefore, if we chose to use enoxaparin in the ED, we need to make sure that it won’t interfere with the ability of the patient to undergo PCI, and that it won’t increase their risk of adverse outcomes. Fortunately, this has been evaluated in controlled trials:
STEEPLE (Safety and Efficacy of Enoxaparin in PCI patients, an international randomized evaluation). This trial examined the safety and efficacy of IV bolus (0.5 mg/kg or 0.75 mg/kg) of LMWH at time of PCI.[7]
Bleeding: Incidence of non-CABG-related bleeding complications in first 48 hours - 5.9% with 0.5 mg/kg enoxaparin (p=0.01 vs. UFH), 6.5% with 0.75 mg/kg enoxaparin (p= 0.051 vs. UFH), and 8.5% with UFH.
Conclusion: IV bolus 0.5 mg/kg enoxaparin associated with reduced rates of bleeding, 0.75 mg/kg associated with similar bleeding risk to UFH. For the combined end point of bleeding and ischemic events, both doses of LMWH were non-inferior to UFH.
1-year mortality rates were comparable between patients receiving enoxaparin and UFH (2.3% for 0.5 mg/kg, 2.2% for 0.75 mg/kg, 1.9% for UFH)
CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of ACC/AHA guidelines). This trial studied the efficacy and safety of LMWH compared with UFH in high risk NSTEMI patients also receiving early GPIIb/IIIa inhibitor therapy.
In patients who underwent PCI within 48 hours the ORs for risk of death and reinfarction were similar for LMWH compared to UFH (OR 0.93, CI 0.67-1.31).
In patients who underwent PCI >48 hours into hospitalization, LMWH therapy was associated with reduced rates of death or reinfarction (OR 0.57, 95% CI = 0.44-0.73) and transfusion (OR 0.66, 95% CI = 0.52-0.84).
Conclusion: Early invasive management with LMWH and GP IIb/IIIa inhibitor in NSTEMI is safe and doesn’t result in increased bleeding complications. In fact, it actually improves outcomes for those who don’t undergo PCI within 48 hours. 9
Take Home Points:
Enoxaparin has been proven to be at least non-inferior and likely even superior to UFH when it comes to reducing the risk of death and MI in the setting of NSTEMI.
Bleeding risk with enoxaparin compared to UFH appears to be equal (with the exception of the Synergy trial).
Enoxaparin is safe and efficacious for use during PCI.
Dialogue should occur between ED providers and interventional cardiologists to ensure their comfort with enoxaparin use and to prevent bleeding complications. If everyone is on board with using enoxaparin, it will likely get your patient anticoagulated more expediently than they otherwise would while waiting for pharmacy to mix up your heparin drip.
Expert Commentary
Dr. Whipple, thank you for an excellent review of the literature supporting the use of lower molecular weight heparin (LMWH) for Non-ST-Elevation Myocardial Infarction (NSTEMI). There are two important points to discuss here before we even talk about LMWH, specifically: (1) What is the evidence behind good ole unfractionated heparin (UFH) in patients with NSTEMI? (2) Based on the evidence for UFH, is there a subgroup of patients who are likely to benefit more than others?
What is the evidence behind good ole unfractionated heparin (UFH) in patients with NSTEMI?
If you look at the AHA/ACC guidelines, UFH is listed as a Class I recommendation. Like any recommendations in guidelines, it is always important to look back at the data behind it. Specifically with respect to UFH, this data is weak but unfortunately is the best we have. Many of studies supporting the use of UFH in NSTEMI involved patients with “unstable angina” in the era before modern laboratory diagnostics (most studies used creatinine kinase), dual anti-platelet therapy (DAPT), GpII/IIIa inhibitors, early invasive strategies, and revascularization [10]. This is a very different population than NSTEMI patients today. In general, these studies did find a strong trend in reduction of composite endpoints (mainly recurrent angina, death or MI) however this was only during hospitalization (short term endpoints) and this is the main crux of the AHA/ACC guideline recommendations. Further in the guidelines favor, the Cochrane review also concluded that UFH in NSTEMI reduces the rate of MI with a relative risk of 0.4 (0.25-0.63) [11]. However what both the guidelines and Cochrane review failed to consider is the benefits of UFH to our patients at a later time point. Deeper analysis of the Cochrane review reveals that the majority of their data points came from the FRISC study, which used only a six-day end point for their outcomes. A meta-analysis performed by Oler in 1996, took into account a time period beyond the UFH treatment duration (2-12 weeks) and found no significant difference in outcomes:
“Because the anticoagulant effects of heparin are brief, any benefit of therapy is unlikely to last beyond the duration of treatment. Consistent with this theory, we found no reduction in the risk of MI or death between 2 and 12 weeks following randomization in patients with unstable angina who received heparin and aspirin compared to those who received aspirin alone. This result underscores that heparin is a short-acting, temporizing therapy, and not an intervention that alters underlying atherosclerotic disease. - Oler et al. 1996”
Not only do the studies that the guidelines base their data off fail to consider more later end points, they also include a different patient population, in a different era of acute coronary syndrome management. Since heparin has now become the standard of care for management of NSTEMI, no further placebo- controlled trials of heparin will ever exist.
Based on the evidence for UFH, is there a subgroup of patients who are likely to benefit more than others?
Based on the discussion above, there isn’t strong evidence to support UFH use in all comers with NSTEMI however based on its mechanism of action, it is likely to benefit those with high risk NSTEMI (TIMI Risk Score), or those who will undergo coronary intervention or revascularization. Intuitively this makes sense and jives with the evidence in the AHA/ACC guidelines. Patients who are placed on UFH and bridged to PCI/revascularization will benefit from the theoretical plaque stabilization. Those who are observed without any intervention will not, as once heparin is discontinued, their ongoing plaque burden and coronary anatomy will be unchanged, placing them at risk for a “rebound” event.
In the emergency department we are burdened with making decisions with minimal initial information and only a few hours of observation. That being said, making a confident decision about which NSTEMI patients will need PCI/revascularization (and therefore will benefit from UFH) may prove very difficult. Therefore it may be more prudent to consider when certain situations make intervention less likely. These situations may include:
Patients without ACS symptoms but with elevated troponin:
Renal disease
hypotension
Sepsis
Toxic ingestion
Significant metabolic derangement
Anemia
Heart failure (without suspicion for ischemic etiology)
Supraventricular tachycardia (SVT)
Other presentations where troponin elevation is suspected to be related to a supply/demand issue
Patients with atypical history and high bleeding risk
The best course of action in these situations is to discuss the utility of UFH with the consulting cardiologist or admitting hospitalist about what is right for your patient based on the risk and benefits of anticoagulation, as well as your clinical suspicion for true acute coronary syndrome.
UFH or LMWH?
Now that we have a better understanding of the utility of UFH in NSTEMI, the use of LMWH becomes more clear. The same considerations just discussed should be similarly applied to the use of LMWH. As your literature review demonstrates, LMWH appears to be as good (if not better) than UFH with a similar bleeding risk profile. It is also easier to administer, requires less monitoring and has a lower risk of accidental supra-therapeutic anticoagulation. While it seems that it may be an obvious decision to switch to LMWH, remember that there is always a significant time lag between evidence and its incorporation into clinical practice. Therefore as you mentioned in your take home points, clear communication with other services is key. Before you go rogue giving LMWH to all your NSTEMI patients, I recommend having a evidence based discussion with your cardiologists and hospitalists to ensure everyone is on the same page.
Michael Macias, MD
NUEM Alumus 2017
University of California, San Diego
How To Cite This Post
[Peer-Reviewed, Web Publication] Whipple T, Ketterer A. (2019, March 18). Lovenox in NSTEMI [NUEM Blog. Expert Commentary by Macias M]. Retrieved from http://www.nuemblog.com/blog/lovenox-NSTEMI
Other Posts You May Enjoy
Resources:
Antman EA, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction results of the TIMI 11B trial. Circulation. 1999; 100: 1593-1601.
Amsterdam EA, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. Circulation. 2014;000:000–000. DOI:10.1161/CIR.0000000000000134
Blazing MA, et al. Safety and efficacy of enoxaparin v unfractionated heparin in patients with no-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA. 2004; 292: 55-64.
Cohen et al. A comparison of Low-Molecular-Weight Heparin with Unfractionated Heparin for unstable coronary artery disease. N Engl J Med. 1997; 337: 447-52.
Harvey et al. Efficacy and safety of enoxaparin compared with unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention in the Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa inhibitors (SYNERGY) trial. Am Heart J 2006; 152: 1042-50. Doi: 10.1016/j-ahj.2006. 08.002
Montalescot et al. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention. N Engl J Med. 2006; 355: 1006-1017. DOI: 10.1056/NEJMoa052711
Montalescot, et al. Enoxaparin versus unfractionated heparin in elective percutaneous coronary intervention: 1 year results from the STEEPLE Trial. J Am Coll Cardiol Intv 2009; 2: 1083-91.
Peterson JL, et al. Efficacy and bleeding complications among patients randomized to enoxaparin or unfractionated heparin for antithrombin therapy in non-ST-Segment Elevation Acute Coronary Syndromes. JAMA. 2004;292:89-96.
Singh KP, et al. Low-molecular-weight heparin compared with unfractionated heparin for patients with non-ST-segment elevation acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE initiative J Thromb Thrombolysis. 2006; 21:211–220
Oler A, et al. Adding heparin to aspirin reduces the incidence of myocardial infarction and death in patients with unstable angina. A meta-analysis. JAMA. 1996; 276(10):811-5.
Andrade-Castellanos CA, et al. Heparin versus placebo for non-ST elevation acute coronary syndromes. Cochrane Database. 2014; (6):CD003462.
Antman EM, et al.The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000; 284(7):835-42.
ECMO Initiation in the ED
Written by: Kaitlin Ray, MD (NUEM PGY-3) Edited by: Evan Davis, MD (NUEM Alum ‘18) Expert commentary by: Colin McCloskey, MD (NUEM Alum ‘16)
ECMO Initiation in the Emergency Department
Introduction:
Extracorporeal membrane oxygenation (ECMO) provides prolonged cardiopulmonary support in severe acute respiratory or cardiac failure. As the science behind ECMO continues to grow and with promising data regarding its use in acute hypoxemic respiratory failure, cardiac arrest, and cardiogenic shock, ECMO use in the United States has increased over 400% in the last ten years. This has stimulated an interest in earlier applications of ECMO both in the emergency department (ED) and even in the prehospital setting [1]. Initiation of ECMO in the ED is a relatively new development, with 65% of programs <5 years old and the majority of programs with <3 cases per year. However, this number continues to grow [2].
There two main types of ECMO: venoarterial (VA) and venovenous (VV). While VV ECMO provides respiratory support, only VA ECMO provides both respiratory and hemodynamic support. ECMO drains blood from the native vascular system, then circulates it outside of the body via a mechanical pump where it passes through an oxygenator and heat exchanger. Hemoglobin then becomes saturated with oxygen, CO2 is removed, and blood then reinfuses into the circulation [4]. Venoarterial ECMO is more commonly utilized in the emergency department as eligible ED patients often have concurrent hemodynamic and respiratory collapse.
Emergency medicine physicians have an increasing responsibility to initiate ECMO and/or make the decision to transfer to an ECMO capable facility. Knowing this, it is critical that we are familiar with the types of ECMO available, understand the indications, contraindications, risks, benefits, and logistics of initiating this form of extracorporeal life support.
Indications/Contraindications:
The specific criteria and contraindications to ECMO vary from institution to institution, often making the decision and ability to initiate ECMO challenging. The Extracorporeal Life Support Organization (ELSO) provides specific guidelines for ECMO initiation. These indications include cardiogenic shock as defined as (1) hypotension and low cardiac output with inadequate tissue perfusion despite adequate intravascular volume and (2) persistent shock despite volume, inotropes, pressors, and possibly an intraaortic balloon pump. The ELSO also provides guidelines for ECMO in acute respiratory collapse, as well as contraindications for ECMO including: unrecoverable heart failure and not a candidate for transplant or LVAD, advanced age, chronic multi-system failure, compliance issues, terminal malignancy and prolonged CPR without adequate tissue perfusion.
As emergency physicians, which patients should we consider as candidates for ECMO? Generally speaking, consider younger and healthier patients who experience an acute but reversible insult leading to rapid cardiopulmonary collapse. Recall that ECMO should only be considered as a bridge to more definitive therapy. Examples of scenarios that fit this criterion include:
Massive pulmonary embolism
Myocardial infarction causing V-Tach/V-Fib or cardiogenic shock
Acute myocarditis or cardiomyopathy causing cardiogenic shock
Drowning
Hypothermia
Drug overdose causing cardiovascular collapse (such as beta-blocker or Ca-channel blocker)
Massive smoke inhalation, pulmonary contusion, or pulmonary hemorrhage causing refractory hypoxemia
As mentioned above, generally those with more chronic conditions such as end-stage heart failure, end-stage COPD, or those with chronic multi-organ failure, do not make good ECMO candidates. Other patient populations to consider in an ICU rather than ED setting include those with septic shock and/or ARDS. Note that patients with traumatic injury leading to hemorrhagic decompensation, although acute in nature, typically are not good candidates for ECMO as ECMO does not prevent further blood loss.
Additionally, we must consider what an ECMO-eligible patient clinically looks like. The majority of the patients who present with one of the above conditions will either be responsive to conventional therapies (intubation, fluids, inotropes), or they will be dead on arrival. However it is the rare, in-between patient that should be considered for ECMO. The condition of a good candidate would include things like:
Persistent hypotension despite maximum conventional therapy
Persistent hypoxemia despite maximum ventilator therapy
Patients brought in in cardiac arrest but achieve periods of unsustained ROSC
Patients brought in with vitals but arrest in the ED
Unfortunately patients who arrest in the field, are brought to the ED already in cardiac arrest, or who do not achieve ROSC despite 30-45 minutes of well executed ACLS are unlikely to be appropriate ECMO candidates. The critical ECMO population is truly those who are flirting with life vs. death, especially the patients with intermittent periods of ROSC. Key exceptions to this include drowning and/or hypothermic patients. Generally these patients are better candidates for ECMO even if there has not been a recorded pulse, with the caveat that they should have been pulled out of the water or other environment quickly.
Risks/benefits:
ECMO is unique in that it provides full cardiopulmonary support without the physical trauma of chest compressions, thereby decreasing trauma, stress, and number of interruptions. Additionally, it provides a higher flow state than would otherwise be provided by manual compressions [2]. VV ECMO also minimizes barotrauma, volutrauma, and oxidative stress. However ECMO is not without risks and complications. The risk of bleeding is significant in the context of continuous anticoagulation and platelet dysfunction. Thromboembolism may lead to stroke or limb ischemia [4]. Infection may also occur secondary to indwelling lines/tubes [1].
Logistics:
ECMO is a costly intervention that requires a multi-disciplinary approach and an organizational commitment in order to proceed. Consideration must be given to the required equipment, blood bank capabilities, cannulation, and personnel availability. In order for ECMO to be successfully initiated from the ED, coordination between EMS, emergency medicine physicians, the cath lab, nursing staff, neurocritical care, cardiothoracic surgery, and the ICU is required [3]. When cannulating for VV ECMO, one may use a two cannula approach (femoral vein and internal jugular/SVC), or a single dual-lumen cannula (right atrium/IVC via the IJ). VA ECMO typically involves cannulation through the femoral artery and femoral vein [1]. If CPR is ongoing during cannulation attempts, programs may use a modified ACLS algorithm with a continuous epinephrine infusion at 0.7 mg/kg/min and minimization of pulse checks by utilizing continuous TEE monitoring [3]. Aggressive anticoagulation is required with continuous infusion of either unfractionated heparin or direct thrombin inhibitor, and efforts should be made to maintain platelet counts >50K and hemoglobin >12 mg/dL6,7.
Recap:
While initiation of ECMO from the emergency department is still a relatively new endeavor for many certified ECMO centers, the ED is in a unique position to bridge select patients in acute respiratory or cardiac failure to recovery using ECMO. While institutional criteria for ECMO varies, the ELSO guidelines may be used as a reference to guide decision making in the absence of formal criteria. Generally speaking, pursue ECMO for younger, healthier patients with acute hemodynamic and/or respiratory collapse that is potentially reversible and unresponsive to conventional therapies. Typically patients with massive PE, MI, hypothermia, drowning, acute cardiomyopathy are the best candidates for ED ECMO. Contraindications generally include severe neurologic injury, end stage malignancy, advanced age, and irreversible multi-organ failure. Knowing that the emergency physicians are often the first to receive patients in acute cardiac and respiratory failure, it is critical that we are familiar with the types of ECMO available, understand the indications, contraindications, risks and benefits, and logistics of initiating this form of extracorporeal life support.
Expert Commentary
This is a thoughtful and thorough overview of ECMO within the emergency department. I will limit my commentary to VA ECMO for cardiopulmonary failure (ECPR), given the enthusiasm for the topic in the FOAM world and my experience within a ED based ECMO program. Some broad themes I would like to highlight: Evidence, Patient selection and Systems of Care.
Evidence: There is a signal that ECPR is better than conventional CPR. A systematic review and metanalysis found that those with cardiac arrest who received VA ECMO had an association with increased neurologically intact survival, with a number needed to treat of 7 [1]. However, most of the data is retrospective and from single centers, making it subject to a number of confounders, as well as selection bias. Further, those who received ECPR were more likely to receive therapeutic hypothermia and percutaneous catheterization, both interventions known to improve outcome following cardiac arrest. Another single center experience has been promising, with 9/18 patients surviving to hospital discharge with good neurological outcome [2]. This protocol involved EMS bypassing the ED and taking the patient to the cardiac catheterization lab where they were placed on ECMO and underwent catheterization. Those who had good outcome all had concomitant intervenable coronary artery disease. There are several centers that have similar experiences with published case series [3,4], but it depends thus far on quality patient selection and a viable system of care.
Patient Selection: All the above trials had strict inclusion and exclusion criteria. Most established protocols include an age cutoff (65-75 depending on center), initial shockable rhythm, and a time from arrest to cannulation between 30-60 minutes. Pertinent exclusions include advanced comorbidities, initial asystole or prolonged downtime. This is done with the intent of cannulating patients with the best chance of surviving their ECMO run; namely young patients with likely coronary artery disease who need ECMO as a bridge to cardiac catheterization. VA ECMO’s other successful ED applications, namely pulmonary embolism [5], drug overdose [6], and acute myocarditis [7] all share the commonality that ECMO provides time for recovery or as a bridge to a viable intervention. A bridge to recovery must exist prior to any cannulation scenario; this cannot be understated.
Systems of care: Cannulation is just one step in a VA ECMO patients hospital course. When conceptualizing a successful ED ECMO program, the institutional commitment should be visualized: A patient requiring 5-7 days in the ICU, formal neuroprognostication and continuous goals of care discussions with family. You rightly include a logistics session in your review, but this system of care is paramount to a successful ECMO program. Prehospital EMS systems must be designed for quick recognition and transport of ECMO candidates. Emergency physicians need to be trained, and maintain competency in ECMO cannulation; interventional cardiology must be willing to catheterize appropriate patients; ICU consultants must have a standardized protocol for post-arrest care and neurology/ICU must have an institutionally accepted neuroprognostication scheme. In parallel to this, the family discussions regarding prognosis and any transition of care should include social work, case management and palliative care professionals. Cannulation is as exciting a procedure an emergency physician can perform, but without a thoughtful, multidisciplinary system of care, these patients will do poorly.
In closing, VA ECMO in the emergency department is an exciting development to tertiary ED practice. More experience, and more data, will help define the niche of patients and the necessities of post-arrest care that provide these patients with the best outcome.
1. Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922-1934.
2. Yannopoulos D, Bartos JA, Martin C, et al. Minnesota resuscitation consortium's advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5(6):10.1161/JAHA.116.003732.
3. Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88-94.
4. Bellezzo JM, Shinar Z, Davis DP, et al. Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation. 2012;83(8):966-970.
5. Yusuff H, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A systematic review. Perfusion. 2015;30(8):611-616.
6. Wang G, Levitan R, Wiegand T, et al. Extracorporeal membrane oxygenation (ecmo) for severe toxicological exposures: Review of the toxicology investigators consortium (toxic). Journal of Medical Toxicology. 2016;12(1):95-99.
7. Nakamura T, Ishida K, Taniguchi Y, et al. Prognosis of patients with fulminant myocarditis managed by peripheral venoarterial extracorporeal membranous oxygenation support: A retrospective single-center study. Journal of intensive care. 2015;3(1):5.
Colin McCloskey, MD
NUEM Alum ‘16, Critical Care Anesthesiology fellow - University of Michigan Medical Center/University of Michigan Health System
How To Cite This Post
[Peer-Reviewed, Web Publication] Ray K, Davis E (2018, November 12). ECMO Initiation in the ED [NUEM Blog. Expert Commentary by McCloskey C]. Retrieved from http://www.nuemblog.com/blog/ECMO
Other Posts You May Enjoy
Resources
Mosier, Jarrod M., et al. “Extracorporeal Membrane Oxygenation (ECMO) for Critically Ill Adults in the Emergency Department: History, Current Applications, and Future Directions.” Critical Care, BioMed Central, 17 Dec. 2015, ccforum.biomedcentral.com/articles/10.1186/s13054-015-1155-7.
Tonna, Joseph E., et al. “Practice Characteristics of Emergency Department Extracorporeal Cardiopulmonary Resuscitation (ECPR) Programs in the United States: The Current State of the Art of Emergency Department Extracorporeal Membrane Oxygenation (ED ECMO).” Resuscitation, Elsevier Ireland Ltd, 10 Sept. 2016, experts.umich.edu/en/publications/practice-characteristics-of-emergency-department-extracorporeal-c.
Tonna, Joseph E., et al. “Development and Implementation of a Comprehensive, Multidisciplinary Emergency Department Extracorporeal Membrane Oxygenation Program.” Annals of Emergency Medicine, Mosby Inc., 1 July 2017, cwru.pure.elsevier.com/en/publications/development-and-implementation-of-a-comprehensive-multidisciplina.
Bartlett, Robert. Extracorporeal Membrane Oxygenation (ECMO) in Adults, 16 June 2017, www.uptodate.com/contents/extracorporeal-membrane-oxygenation-ecmo-in-adults.
Extracorporeal Life Support Organization - ECMO and ECLS. “Guidelines for Adult Respiratory and Cardiac Failure.” Extracorporeal Life Support Organization - ECMO and ECLS > Resources > Guidelines, ELSO (Extracorporeal Life Support Organization), Dec. 2013, www.elso.org/Resources/Guidelines.aspx.
Sklar MC, Sy E, Lequier L, et al. Anticoagulation Practices during Venovenous Extracorporeal Membrane Oxygenation for Respiratory Failure. A Systematic Review. Ann Am Thorac Soc 2016; 13:2242.
Spinelli E, Bartlett RH. Anemia and Transfusion in Critical Care: Physiology and Management. J Intensive Care Med 2016; 31:295.
Approach to Hypothermic Resuscitation
Written by: Luke Neil, MD (NUEM PGY-2) Edited by: Quentin Rueter, MD, (NUEM PGY-4) Expert commentary by: Kory Gebhardt, MD
Expert Commentary
This is a good overview of the algorithmic approach to the hypothermic patient. Generally speaking, hypothermia can be divided into various categories of severity, but as you mention, it is really those patients with a core temperature of <32°C (90°F) with cardiac instability or cardiac arrest that will require especially aggressive care.
For any hypothermic patient, the most important initial intervention is to stop any further heat loss. This is especially important for those with damp or wet clothing. Any wet garments should be completely removed, the patient should be dried, and then covered with warm, dry blankets and possibly a forced air rewarming device (i.e. Bair Hugger). Recall that one of the most efficient ways to cool a HYPERthermic patient is with evaporative cooling (spraying with or submerging them in water and then using fans to circulate air over the wet surfaces). Similarly, this heat loss will strongly work against you in rewarming a hypothermic patient if they are not fully dry. After this simple intervention, the majority of mildly hypothermic and stable patients just need time to bring their core temperature back to normal and often can be discharged once this has occurred.
For those patients with a core temp >32°C with severe cardiac instability or in cardiac arrest, you should also consider alternative etiologies for their presentation rather than expect it solely caused by the hypothermia alone. Like you mention, if you are able to rewarm a cardiac arrest patient above this temperature and they remain in asystole, it is likely that irreversible damage has occurred and they are less likely to be able to be successfully resuscitated.
As you detail in the algorithm, those with a temperature less than 32°C (90°F) AND instability or arrest need aggressive and invasive rewarming. The best available means of doing this is ECMO. Much of the research surrounding accidental hypothermia and resuscitation comes from the Nordic countries where freezing temperatures are often combined with outdoor extracurriculars and results in a high “n” for the studies. Outcomes data from many of the expert centers in this area show major benefits of ECMO, including one showing survival post-arrest in nearly 60% of patients and, even more importantly, good neurologic outcomes in 38% compared to only 3% in those without extracorporeal rewarming!
Unfortunately, not all EM physicians will have quick or 24/7 availability of ECMO. While this should be the preferred means of rewarming if available, there are alternatives if it is not. Hemodialysis circuits can also be used to actively rewarm a patient. Generally these can achieve 2-4 degrees/hr of rewarming compared to the 4-6 degrees/hr of ECMO. Thoracic (bilateral chest tubes), gastric (NG tube), and bladder lavage (foley) with warm fluids can also provide several degrees per hour of rewarming if used appropriately. Use a ventilator that can warm and humidify air. Don’t forget about minimizing heat loss by fully drying the patient and keeping as much of them covered as possible.
Lastly, I want to say a word about prognostication. While the mantra is, “you’re not dead until you’re warm and dead”, you can imagine that these patients require a considerable amount of time, effort, and mobilization of resources when they present to the ED. There is information that can help guide which patients are likely to benefit from such aggressive care from those who are, unfortunately, unlikely to be resuscitated. While multiple markers have been studied, the one with the most evidence supporting it, is a potassium value. This value can serve as a sort of surrogate for “warm ischemia time”, or in other words, how long were they warm and dead. This should be obtained and sent early in the resuscitation of the patient. If the value is >12, there is nearly no chance of any meaningful recovery (still very unlikely at >10, and even a cutoff of >8). Conversely, if the potassium level is less than the 8-12 range, the patient still has a good chance at a meaningful recovery if resuscitated to ROSC and these are the patients that should receive everything we have to rapidly and efficiently rewarm them (they are also the patients that can have meaningful recoveries despite impressive downtimes of even hours).
Additionally, historical factors surrounding the hypothermia, if known, can provide valuable prognostic information. Immersion vs. Submersion, which you define in your algorithm, is one example that might influence your decision about whether a patient might have benefit from mobilizing ECMO or other aggressive/invasive rewarming.
Kory Gebhardt, MD
Kaiser Permanente Emergency Medicine
How to cite this post
[Peer-Reviewed, Web Publication] Neil L, Rueter Q (2018, June 4 ). Approach to Hypothermic Resuscitation. [NUEM Blog. Expert Commentary by Gebhardt K]. Retrieved from http://www.nuemblog.com/blog/hypothermia
Treatment of pSVT: A Case for Calcium Channel Blockers
Written by: Amanda Randolph, MD (NUEM PGY-1) Edited by: Jim Kenny, MD, (NUEM PGY-4) Expert commentary by: Meghan Groth, PharmD - Emergency Medicine Clinical Pharmacist, UMass Memorial Medical Center
The Case
A 37 year-old woman presents to the ED for palpitations. On the monitor, you see her heart rate is 190, but all other vitals are within normal limits. She feels anxious but is otherwise asymptomatic, breathing comfortably on room air. The rest of the physical exam is unremarkable. The patient tells you, “I think it’s my SVT again - I was just here for this last month!”
Her rhythm strip looks something like this:
https://lifeinthefastlane.com/ecg-library/svt/
SVT generally refers to any tachyarrhythmia generated above the His/Purkinje system. For simplicity, the term pSVT in this post will refer to only Atrioventricular Nodal Tachycardia (AVNRT), as it is the most common tachyarrhythmia in patients with normal cardiac structure [1].
SVT: Treatment Guidelines
You double-check the current ACLS protocol (2015) for the treatment of pSVT [2]:
Figure 1. ACLS 2015 guidelines for treatment of AVNRT
This patient is stable, so you try some vagal maneuvers, including carotid massage and Valsalva. You even try the modified Valsalva maneuver you read about in the REVERT trial (straining followed by leg elevation and supine positioning), which is described to have a 43% success rate.
Despite your best efforts, the vagal maneuvers fail, so you ask the nurse to draw up some adenosine.
At this point, the patient yells, “Absolutely no way! I’m not trying Adenosine - it makes me feel like I’m going to die! There has to be something else.”
You know Calcium Channel Blockers (CCBs) are recommended as a second line drug if adenosine does not terminate the SVT, or if adenosine is contraindicated. But what does the data say? Is it ever reasonable to jump straight to CCBs?
The Problem with Adenosine
Adenosine administration is widely recognized to produce a variety of minor side effects, as listed below4. While not quantified in any studies to date, these “minor” side effects can be extremely traumatic for patients. This distress can have lasting psychological effects that may delay or even prevent patients from seeking care [5].
- Chest pain (7-40%)
- Facial flushing (18-44%)
- Nausea (13%)
- Headache (2-18%)
- Lightheadedness/Dizziness (12%)
The Problem with Calcium Channel Blockers
Current ACC/AHA guidelines give CCBs a class IIa recommendation for use in pSVT [2]. However, most EM practitioners continue to favor Adenosine, in part because of cultural dogma, but also due to concern about inadequate data to regarding the efficacy and safety for calcium channel blocker use.
One pharmacologic difference between CCBs and Adenosine is the onset of action (100-400 seconds for CCBs compared with 21-34 seconds for Adenosine), which can create a delay to conversion [5]. However, because CCBs are only used in stable patients, this slightly longer onset is unlikely to be clinically significant [6].
More importantly, one of the most feared side effects of Calcium channel blockers is hypotension, as CCBs work by creating negative inotropy and peripheral vasodilation. In one study by Lim et al., the change in blood pressure after administration of adenosine was -2.6/-1.7, compared to -13.0/-8.1 with verapamil [9].
Of note, the duration of action is quite long for CCBs (2-5 hours), compared with adenosine (<10 seconds).7 This raises a concern that hypotension and other adverse effects of CCBs may be prolonged. For this reason, CCBs are contraindicated in patients with severe HFrEF.6,7 Additionally, CCBs are relatively contraindicated in patients taking beta blockers, as the combined effect can cause significant bradycardia and even heart block [6].
Theoretically, the use of CCBs via slow infusion instead of IV bolus may reduce the risk of hypotension,8 though there is limited data to support this. One randomized trial by Lim et al. compared the use of adenosine (n = 104) vs slow infusion of verapamil (n = 48) or diltiazem (n = 54), and reported no difference in outcomes between adenosine bolus and slow infusion of verapamil or diltiazem [9].
Calcium Channel Blockers vs. Adenosine - The Data
To date, there have been three meta-analyses comparing the efficacy and safety of CCBs to adenosine in patients with pSVT, including a recently published Cochrane review in October 2017.5,6,10 Note that the data described in these studies only refer to the use of Verapamil. Their findings are depicted below (table design inspired by a phenomenal ALiEM post) [8].
A Few Notes on Hypotension after Verapamil:
- None of these metaanalyses specifically reported their definition of hypotension, nor did they clarify whether any of these patients had clinical signs of shock.
- Holdgate and Foo reported two of three hypotensive patients subsequently reverted with adenosine and did not require any other specific treatment for their hypotension (the outcome and interventions for the third case were not reported).
- The study by Lim et al. using slow infusion of verapamil reported only one patient with clinically significant hypotension, with a drop in blood pressure from 122/81 mmHg to 74/61 mmHg after 7.5 mg of verapamil infusion. This patient’s SVT was terminated by synchronized cardioversion, after which his blood pressure improved to 103/69 mmHg.
Case Resolution
After the vagal maneuvers, you give 5mg IV Verapamil. The patient remains stable and converts to sinus tachycardia. She tells you she prefers Verapamil to Adenosine and will be “much less afraid” to come in next time.
Conclusion
Overall, both Adenosine and Verapamil are reasonable choices for termination of SVT. Anecdotally, some patients prefer Verapamil; however, there is limited evidence to support this [6]. Given the current data, physicians should discuss the pros/cons of each drug with the patient and employ shared decision-making when possible.
Take Home Points
- Start with vagal maneuvers, especially the modified Valsalva
- Adenosine and Verapamil are equally effective for SVT
- Moderate evidence by recent Cochrane review
- Class IIa by ACC/AHA
- Adenosine has a much higher incidence of minor side effects
- chest pain, facial flushing, nausea, headache, and lightheadedness/dizziness
- Verapamil has a slightly higher risk of hypotension
- Verapamil: -13/-8 mmHg; Adenosine -2.6/-1.7 mmH
- Rarely clinically significant - cases reportedly resolved with adenosine or synchronized cardioversion
- Always employ shared decision-making when possible
Expert Commentary
Thank you for your insightful post on this all-too-common conundrum we face in the ED. I think it’s incredibly important to remember, as you point out, that treatment of pSVT in the ED doesn’t have to be a “one size fits all” approach, and that we have more than just adenosine available as a treatment agent.
Most of the data for CCBs in this indication is with verapamil, but I’ve become comfortable recommending diltiazem in its place due to a lower risk of hypotension (see post for reference).
When attempting to mitigate the potential hypotension associated with calcium channel blockers, the study by Lim and colleagues that you mentioned is worth noting in more detail. Rather than the traditional 0.25 mg/kg diltiazem bolus (with 0.35 mg/kg repeat dose), subjects instead received diltiazem at a rate of 2.5 mg/min for up to 20 minutes (max dose 50 mg). This approach can optimize dose, reduce potential for hypotension, and spare the patient that “impending doom” feeling often experienced with adenosine (see further discussion on this here).
There are also some cases when adenosine should not be routinely administered, such as patients with reactive airway disease at risk of bronchospasm. A more thorough review of this topic is presented here but in such cases calcium channel blockers represent a reasonable alternative.
The strategy of using calcium channel blockers for pSVT can perhaps leave providers wanting in terms of the instant gratification that comes with adenosine administration, but agents like diltiazem or verapamil have demonstrated efficacy while avoiding some of the unpleasantries of adenosine.
For me, it comes down to recognizing that adenosine isn’t the only drug we have available for the treatment of pSVT. Calcium channel blockers like diltiazem may be used, and if we decide to try them, we can use different dosing approaches such as the slow bolus method outlined above to reduce some of the potential side effects.
Meghan Groth, PharmD
Emergency Medicine Clinical Pharmacist
UMass Memorial Medical Center
[Peer-Reviewed, Web Publication] Randolph A, Kenny J (2018, May 28 ). Treatment of pSVT: A case for calcium channel blockers. [NUEM Blog. Expert Commentary by Groth, M]. Retrieved from http://www.nuemblog.com/blog/PSVT
Posts you may also enjoy
References
- Burns, E., Supraventricular Tachycardia (SVT), in Life in the Fast Lane, M. Cadogan and C. Nickson, Editors. 2012.
- Page R, Joglar J, Caldwell M, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):e27-e115.
- Appelboam, A., et al., Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. The Lancet, 2015. 386(10005): p. 1747-1753.
- Adenosine. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com. Accessed November 12, 2017.
- Delaney, B., J. Loy, and A.-M. Kelly, The relative efficacy of adenosine versus verapamil for the treatment of stable paroxysmal supraventricular tachycardia in adults: a meta-analysis. European Journal of Emergency Medicine, 2011. 18(3): p. 148-152.
- Alabed S, Sabouni A, Providencia R, Atallah E, Qintar M, Chico TJA. Adenosine versus intravenous calcium channel antagonists for supraventricular tachycardia. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No.: CD005154. DOI: 10.1002/14651858.CD005154.pub4.
- [Peer Reviewed, Web Publication] S. Brubaker and B. Long (2017 Feb 1). Treatment of Refractory SVT: Pearls and Pitfalls. [EmDocs.net, Expert Commentary by A. Koyfman]. Retrieved from http://www.emdocs.net/treatment-refractory-svt-pearls-pitfalls/
- [Web Publication] S. Rappaport and M. Groth (2016 Mar 3). Calcium channel blockers for stable SVT: A first line agent over adenosine? [AliEm Blog]. Retrieved from https://www.aliem.com/2016/03/calcium-channel-blockers-stable-svt-alternative-to-adenosine/
- Lim S, Anantharaman V, Teo W, Chan Y. Slow infusion of calcium channel blockers compared with intravenous adenosine in the emergency treatment of supraventricular tachycardia. Resuscitation. 2009;80(5):523-528.
- Holdgate, A. and A. Foo, Adenosine versus intravenous calcium channel antagonists for the treatment of supraventricular tachycardia in adults. The Cochrane Library, 2006.
The PESIT Trial: Do All Syncope Patients Need a PE Workup?
Written by: Alex Ireland, MD (NUEM PGY-2) Edited by: Josh Zimmerman, MD, (NUEM class of 2017) Expert Commentary by: D. Mark Courtney, MD
Syncope is defined as a transient loss of consciousness and postural tone caused by cerebral hypoperfusion. This chief complaint is familiar to most emergency physicians given it accounts for more than 1-2 million patient visits in the US annually.[1] The differential diagnosis is broad and crosses multiple organ systems, including, but not limited to, cardiovascular, neurovascular, and hemorrhagic/hematologic causes. Pulmonary embolism (PE) is one cardiovascular cause that is considered to be infrequent. This is because when associated with syncope, it is often made more clinically apparent by signs and symptoms such as dyspnea, chest pain, tachycardia, hypotension, and hypoxemia. The American Heart Association consensus statement on the evaluation of syncope gives little attention to diagnostics for PE, instead focusing on arrhythmia, ischemia, and structural heart disease. [2] We know from a San Francisco Syncope Rule validation trial that when adverse events occur in the minority of patients with generalized syncope, they are related mostly to cardiac arrhythmias, followed by MI- not PE. [3] Previous studies have cited the prevalence of pulmonary embolism as a cause of syncope in hospitalized patients as low as 1.6%. [4] But are we underestimating and under-diagnosing this potentially lethal condition? The PESIT study sought to systematically assess the prevalence of pulmonary embolism in patients admitted for syncope. [5]
“Prevalence of Pulmonary Embolism among Patients Hospitalized for Syncope. Paolo Prandoni, M.D., Ph.D., Anthonie W.A. Lensing, M.D., Ph.D., Martin H. Prins, M.D., Ph.D., Maurizio Ciammaichella, M.D., Marica Perlati, M.D., Nicola Mumoli, M.D., Eugenio Bucherini, M.D., Adriana Visonà, M.D., Carlo Bova, M.D., Davide Imberti, M.D., Stefano Campostrini, Ph.D., and Sofia Barbar, M.D., for the PESIT Investigators*. N Engl J Med 2016; 375:1524-1531. October 20, 2016”
What was the PESIT trial?
Figure 1. Inclusion and Exclusion Flowchart
The PESIT trial was a cross-sectional study of patients older than 18 years of age who were hospitalized for a first episode of syncope. This was a multicenter trial, taking place in 11 hospitals (2 academic, 9 nonacademic) in Italy. There were several important exclusion criteria to this study including patients on anticoagulation, pregnant patients, and those with previous syncopal episodes. Most importantly, however, the study excluded all patients discharged and solely focused on those admitted for an inpatient evaluation (Figure 1).
All included patients were subjected to a standardized history aimed at suggesting the cause of syncope and identifying risk factors for pulmonary embolism (Figure 2). They then underwent mandatory chest radiography, electrocardiography, arterial blood gas testing, and routine blood testing, including a D-dimer assay. Further diagnostic workup for causes other than pulmonary embolism was not standardized and varied between patients based on attending physician discretion.
Figure 2. Standardized History Questions
The presence or absence of PE was assessed with a validated algorithm based on pretest clinical probability and the result of the D-dimer assay. The pretest clinical probability was defined according to the simplified Wells score, which classifies PE as being “likely” or “unlikely” (Table 1). In patients who had a high pretest clinical probability, a positive D-dimer assay, or both, computed tomographic pulmonary angiography or ventilation-perfusion lung scanning was performed.
Outcomes
The primary outcome of the PESIT trial was to measure the prevalence of pulmonary embolism in patients admitted to the hospital for syncope. Secondarily, the thrombotic burden was assessed by measuring the most proximal location of embolus or the percentage of perfusion defect area.
In 58.9% (339/560) of patients, PE was ruled out by low pre-test probability and a negative D-dimer. Of the remaining patients, 42.2% (97/229) had a pulmonary embolism. In the entire cohort, the prevalence of PE was 17.3% (97/560). Thus, the authors concluded that nearly one of every six patients hospitalized for a first episode of syncope has a pulmonary embolism (Figure 3, Table 2).
Figure 3. Results Flowchart
Strengths and Weaknesses of the Study
The major strength of the PESIT trial is the systematic approach with which PE's were diagnosed. ALL admitted patients underwent consideration of and testing for PE based on risk factors and pretest probability. Even if, for example, the initial ECG suggested an arrhythmia as the cause of syncope, if they did not rule out for PE, they went on to get a scan. This ensured a complete and thorough investigation. Furthermore, their results were internally valid, as the prevalence of PE was consistently 15-20% across all 11 centers.
Additionally, the population studied is also fairly representative of those who most emergency medicine physicians would admit to the hospital with syncope. Reasons for admission included trauma, severe comorbidities, failure to identify a cause in the ED, and a high probability of cardiac syncope. While we don’t commonly site the EGSYS score that they used, the components such as palpitations, heart disease, and an exertional component to syncope, are all considerations that factor into our clinical gestalt for admission.
A glaring problem with this study is the significant variation in diagnostic workup beyond PE. Other potential explanations for syncope were much more likely to be undetermined in those with confirmed embolism (see table 2). Because further workup was left to the discretion of individual physicians, alternative and perhaps more causative causes of syncope may have been under-diagnosed or underreported.
Furthermore, this study has limited external validity when used to assess our ED population as a whole, given the exclusion criteria of all patients discharged from the ED. When recalculating the prevalence based on all patients who presented to the ED, only 1 in 26 (97/2584 = 3.8%) patients were diagnosed with a PE, far fewer than their conclusive 1 in 6. A major contributing factor is likely age. As expected, the mean age of patients discharged was much younger at 54 years (range 16 to 79). These patients are much less likely to develop PE based on currently available decision rules such as the PERC Rule.
We commonly use tools such as the PERC Rule or the Wells score to quantify our pretest probability because they include characteristics known to be associated with PE. As seen in the clinical characteristics described in table 2, patients diagnosed with pulmonary embolism had a high prevalence of symptoms commonly associated with PE, such as hypoxemia and tachycardia, or clinical manifestations of DVT. They were also far more likely to have a history of previous venous thromboembolism or to have active cancer. A large proportion of PESIT patients diagnosed with PE presented exactly as we would expect patients with a PE to present. These data strengthen support for current clinical practice, which for most physicians means only entering the diagnostic pathway for pulmonary embolism when history and physical exam suggests it as a potential diagnosis.
Lastly, the PESIT trial spends significant effort quantifying radiographic burden of pulmonary embolism across all their positive cases. As mentioned earlier, all patients were evaluated for PE, regardless of clinical gestalt. However, we must remember that degree of radiographic filling defect does not necessarily correlate with clinically significant pulmonary embolism. In 40% of patients with PE, the extent of pulmonary vascular obstruction was at the segmental or subesegmental level or was less than 25% of total lung area. Some of these may have been clinically insignificant, not likely to have caused the syncopal event, and perhaps were discovered incidentally. A better predictor for the severity of PE might have incorporated factors such as heart rate, systolic blood pressure < 100 mmHg, elevated BNP, and elevated troponins, which are used in the simplified PE Severity Index and a subsequently developed composite score to predict mortality from PE. [6,7}
Take Home Message
In summary, Pulmonary embolism is an important “must-not miss” cause of syncope, but it is likely an uncommon diagnosis in patients who pass out, recover, and appear well – the main way that most patients in the US with syncope present. Overall, 1 in 26 patients who present to the ED with first time syncope may have a PE. A large portion of these may be clinically insignificant and not causative of syncope. Those with symptoms and signs of PE are more likely to have a significant PE and should be evaluated as such. Thus, we should not change our current clinical practice based on the results of the PESIT trial.
Expert Commentary
Internal validity:
Dr. Ireland’s review of the Prandoni study reviews it’s methods and results quite well. He also points out the important ways in which we as consumers of literature should address a study. The first is with respect to internal validity (how well the authors measured what they in fact intended to measure). This takes into account potential for bias, or systematic ways in which error could be introduced into the measurement. Dr. Ireland does not seem to identify many problems with this study from that standpoint. In general I agree with this, if the goal of measurement of this study was to exhaustively test all patients who are admitted largely at the discretion of their doctors after a first event of syncope. If so, then the methods of this paper seem to have resulted in reasonable internal validity. However if the goal of the paper is to identify the prevalence of symptomatically significant PE among ALL patients with first time PE, then it is quite possible that there is bias due to the methods of inclusion. Specifically, the fact that only admitted patients were included, and those admitted had a fairly large degree of comorbid conditions may have resulted in a measurement of PE that is higher than what would be expected for in general “syncope.”
External validity:
The second key question for a consumer of medical literature to ask is, simplistically, “are these patients like mine?” The answer to this is probably no. The patients included are different than a typical US ED "undifferentiated passing out patient" not just because they are Italian, but also because they had a high prevalence of symptoms to suggest PE such as history of PE/DVT, or history of malignancy. In the US, we would probably not consider many of these patients to be “undifferentiated syncope.” Rather, we would consider them to be possible symptomatic PE patients and simply test them. Dr. Ireland points this out in his evaluation of table 2. It is not a novel finding that many of the patients with these comorbidities, signs, and symptoms went on to have PE when tested (Courtney Annals of EM Annals of Emergency Medicine 2010;55(4):307–315).
Another way to examine generalizability is to examine the course of these syncope patients and ask if this is similar to the course that would be taken in the US. Of the 2584 ED syncope patients in this study, 1867 were discharged. Are we discharging 72% of our syncope patients? Whether or not we should be is another question. However, it is likely that the US ED environment has a much lower threshold for admission for syncope than the Italian setting, similar to the way that the US ED environment has a much lower threshold for testing for PE than the European setting. Therefore, it is highly likely that, at least to some extent, these Italian syncope patients are more ill than the average US ED syncope patient. This is supported by the elderly median age in the Prandoni study of 80….meaning half of all their patients were over 80 years of age! Also note that of the 717 remaining patients not discharged, a further 157 were excluded. So this sample really is a unique selected group…..making it difficult to generalize this study to the average US ED patient with syncope.
Dr. Jeff Kline and I explored the possible external generalizability of this report in a re-examination of the PERC dataset which included 7940 patients from 12 US emergency departments, all of whom had symptoms prompting testing for PE. Among 466 PE positive patients, 6.6% reported syncope, while among 7474 PE negative patients 6.0% reported syncope (95% CI for difference, -1.7 - 3.0). This suggested syncope was not a predictor of PE. We also noted in the Prandoni study a mean age of 75 and a high prevalence of main pulmonary artery clot (42%), something we have not found in US studies of undifferentiated PE patients where median percent obstruction was 9%.
Bottom line:
In elderly syncope patients with some combination of tachycardia, tachypnea, hypotension, active cancer, and perhaps especially those without a clear suspected cause of syncope, PE should be a consideration that warrants testing. Perhaps this should be considered even when patients do not have the more traditional symptoms of PE such as dyspnea or chest pain. However, we would caution clinicians NOT to interpret this study as rationale for widespread testing on all or nearly all US ED syncope patients. The outcome of such a simplistic interpretation of this study would undoubtedly result in further radiation and contrast burden and harms for our patients.
D. Mark Courtney, MD
Associate Professor of Emergency Medicine and Medical Social Sciences, Northwestern Emergency Medicine
Posts You May Also Enjoy
How to cite this post
[Peer-Reviewed, Web Publication] Ireland A, Zimmerman J (2017, Sep 27). The PESIT Trial: Do All Patients Need a Syncope Workup? [NUEM Blog. Expert Commentary By Courtney DM]. Retrieved from http://www.nuemblog.com/blog/PESIT
Resources
1. Syncope Evaluation in the Emergency Department Study (SEEDS): A Multidisciplinary Approach to Syncope Management. Win K. Shen, Wyatt W. Decker, Peter A. Smars, Deepi G. Goyal, Ann E. Walker, David O. Hodge, Jane M. Trusty, Karen M. Brekke, Arshad Jahangir, Peter A. Brady, Thomas M. Munger, Bernard J. Gersh, Stephen C. Hammill and Robert L. Frye. Circulation. 2004;110:3636-3645.
2. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Strickberger SA, et. Al. American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group. American College of Cardiology Foundation.; Heart Rhythm Society.; American Autonomic Society. Circulation. 2006 Jan 17;113(2):316-27. No abstract available. Erratum in: Circulation. 2006 Apr 11;113(14):e697.
3. Prospective validation of the San Francisco Syncope Rule to predict patients with serious outcomes. Quinn J, McDermott D, Stiell I, Kohn M, Wells G.. Ann Emerg Med. 2006 May;47(5):448-54.
4. Etiology of syncope in hospitalized patients. Saravi M, Ahmadi Ahangar A, Hojati MM, Valinejad E, Senaat A, Sohrabnejad R, Khosoosi Niaki MR. Caspian J Intern Med. 2015 Fall;6(4):233-7.
5. Prevalence of Pulmonary Embolism among Patients Hospitalized for Syncope. Prandoni P, Lensing AW, Prins MH, Ciammaichella M, Perlati M, Mumoli N, Bucherini E, Visonà A, Bova C, Imberti D, Campostrini S, Barbar S; PESIT Investigators. N Engl J Med. 2016 Oct 20;375(16):1524-1531.
6. Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383.
7. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718.