Most emergency medicine physicians have ready access to their mobile phone and camera. This article reviews how best to utilize photography at the bedside to capture visually essential components of the physical exam to upload into the chart and share with other providers.
Patellar Dislocations
How to identify, treat, and continue to manage patellar dislocations. Outlined by Emergency Medicine Residents and commented on by attending faculty at Northwestern Memorial Hospital.
Massive Transfusion Protocol
An overview of Massive Transfusion Protocol
TXA in the Trauma Bay
Written by: Jilan Shimberg, MD (NUEM ‘26) Edited by: Rafael Lima, MD (NUEM ‘23)
Expert Commentary by: Matthew R Levine, MD
Expert Commentary
Unlike many of the treatments and interventions we use in the Emergency Department and the trauma bay, tranexamic acid (TXA) has rather robust studies to guide usage. Like many interventions, however, even when there are studies with large numbers of patients and positive results, there are still barriers towards implementation. TXA is no different.
Working at a Level 1 Trauma Center and frequently interacting closely with the trauma surgeons through the Trauma Quality Management Committee, I often follow their lead when it comes to promising trauma innovations through the years such as TXA, REBOA, permissive hypotension, and so on. What I have observed is that our trauma surgeons tend to believe that there is benefit to properly timed TXA in the right trauma patients and that we do not use it enough. Yet use of TXA in trauma at our hospital has not been protocoled.
Why not? Some possibilities:
Someone usually (but not always) thinks to give it to patients who would benefit despite there not being a protocol (thanks ED pharmacists!).
The patients who need it most also need something else even more – source control of hemorrhage. Anything that slows or distracts from that may be counterproductive. It may not seem like a simple TXA infusion would delay anything. But keep in mind the multiple lines sick trauma patients may need and the often already chaotic nature of “the bay” getting the sickest patients the tubes, meds, lines, products, studies, and, ultimately, proper disposition during their “golden hour”. The nurses have many tasks, to say the least. But maybe this is an argument for why use of TXA should just be protocolized.
I bounced this off of our trauma section head to make sure I was not misrepresenting their thoughts. As a result, we are looking into protocolling its use. Thanks NUEM Blog!
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Shimberg, J. Lima, R. (2024, Mar 11). TXA in the Trauma Bay. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/txa-trauma
Other Posts You May Enjoy
Traumatic Arthrotomy
Written by: Parisa Kermani, MD (NUEM ‘23) Edited by: Alex Herndon, MD (NUEM ‘21)
Expert Commentary by: Matt Levine, MD
Case: A 25-year-old male comes into the ER after a saw accident at work. The patient was using a circular saw to cut wood when it slipped and the saw touched up against his knee. The patient has a 10cm linear vertical laceration over the anterior surface of his left knee (Figure 1). Bleeding is controlled. Patient ambulatory. Reporting 10/10 pain over the laceration.
What are the next best steps for evaluation and treatment of his injury?
Figure 1: Knee laceration
Background
Traumatic arthrotomy is defined as a soft tissue injury over a joint that penetrates the joint space. Violation of the joint capsule exposes the sterile intra-articular space to the environment which can result in a deep infection and sepsis. The morbidity associated with septic arthritis is high, so it is important that providers have a high index of suspicion when evaluating wounds over joint surfaces.
The knee joint is the most common joint to be affected, followed by the ankle. Penetrating injuries have a higher rate of capsule violation so a history of knives or bullets should raise suspicion, though MVCs, falls, motorcycle accidents can also result in a deep injury. The capsule has little protection lateral to the patella (Figure 2 & 3), so even if the laceration does not appear deep there is potential that it penetrates the joint space.
Figure 2: Knee capsule anatomy
Figure 3: Knee CT scan
Evaluation
Exploration: The first step of evaluation is local wound exploration. It is useful to anesthetize the wound at this point, as this will make the patient more comfortable and allow for a better exam. Irrigate the wound with sterile saline. It is extremely important to visualize the base of the wound. Using a hemostat or q-tip to probe the tissue at the base can be helpful as to not miss any tunneling segments. Keep a close eye out for bubbles, synovial fluid (appears straw colored and oily) or visible bone/tendon as all of these indicate joint involvement. It is important to note that the absence of these findings does not rule out a traumatic arthrotomy.
X-ray: Many times, next step will be to get an X-ray to look for associated fractures. Though this is not the most sensitive test for evaluating for joint space violation, if you see intra-articular air, this signifies joint involvement and no further imaging is required before calling the orthopedic surgeons. Many times, the X-ray will be normal and further testing will need to be completed. Of note, an x-ray is not required if there is no concern about injury to the bone as it is unlikely going to give a definitive answer on traumatic arthrotomy in less obvious cases.
CT Scan: As far as imaging goes, CT scan is the imaging modality of choice for traumatic arthrotomy. Though not currently the gold standard for ruling out joint violation, CT scan has become more accepted as an alternative to saline load testing the joint. Although limited, a 2013 study by Konda et al, where direct arthroscopic visualization or septic arthritis at follow-up were used as the gold standard for diagnosis, found imaging by CT scan to be 100% sensitive and specific for diagnosing traumatic knee arthrotomy. When viewing a CT scan to evaluate for traumatic arthrotomy, the presence of gas in the joint, known as pneumarthrosis, indicates intra-articular extension (Figure 4).
Figure 4: Traumatic arthrotomy on CT scan
Source: Konda et al, 2013
Saline Load Test (SLT): Though not strongly backed by the literature, SLT is a standard tool used to assess for traumatic arthrotomy. SLT is done by performing an arthrocentesis of the affected joint away from laceration, once confirmed in the correct space, sterile saline is injected into the joint and the laceration site is observed for extravasation. The provider should also passively range the joint while injecting to ensure greater sensitivity. Table 1 below summarizes how much sterile saline should be injected to obtain 95% sensitivity for traumatic arthrotomy. Adding methylene blue to the saline has not been proven to increase sensitivity and generally no longer recommended. The sensitivity will be highly variable based on provider experience with the procedure and patient tolerance. It is important to remember that this procedure can be exquisitely painful and special attention should be paid towards the patient’s comfort.
Table 1: Amount of saline for 95% sensitivity SLT
Because strong, conclusive literature is lacking, the choice between CT versus SLT to rule out traumatic arthrotomy will depend on many different factors including provider procedural comfort, local practice patterns, available resources and patient input.
Treatment
Once a diagnosis of traumatic arthrotomy is confirmed through an above modality, orthopedics should be emergently consulted. Tetanus prophylaxis should be updated and the patient should be started on an IV antibiotic that covers both strep and staph. A 1st generation cephalosporin is usually sufficient. Other antibiotics should be considered if injury is from a human/animal bite, happened underwater, or if there is concern for fecal/other contamination. Definitive treatment is joint wash out in the Operating Room.
If the above modalities do not show evidence of arthrotomy the patient’s laceration may be repaired in usual fashion. The patient should be given strict return precautions and have close follow-up for wound/joint reevaluation and suture removal.
Sources
Browning BB, Ventimiglia AV, Dixit A, Illical E, Urban WP, Jauregui JJ. Does the saline load test still have a role in the orthopaedic world? a systematic review of the literature. Acta orthopaedica et traumatologica turcica. 2016;50(6):597-600. doi:10.1016/j.aott.2016.01.004
Gittings D, Dattilo J, Fryhofer G, Martin A, Hast M, Mehta S. The saline load test is effective at diagnosing traumatic arthrotomies of the shoulder. Journal of surgical orthopaedic advances. 2019;28(4):268-271.
Gittings DJ, Fryhofer GW, Hast MW, Steinberg DR, Levin LS, Gray BL. The saline load test is effective at diagnosing traumatic arthrotomies of the wrist. Techniques in hand & upper extremity surgery. 2019;23(2):59-61. doi:10.1097
Jonathan Michael Strong. Saline Load or CT: What’s the Best Test for Traumatic Arthrotomy. Acepnow magazine. 2020; https://www.acepnow.com/article/saline-load-or-ct-whats-the-best-test-for-traumatic-arthrotomy
Konda SR, Howard D, Davidovitch RI, Egol KA. The saline load test of the knee redefined: a test to detect traumatic arthrotomies and rule out periarticular wounds not requiring surgical intervention. Journal of orthopaedic trauma. 2013;27(9):491-497. doi:10.1097/BOT.0b013e31828211f3
Konda SR, Davidovitch RI, Egol KA. Computed tomography scan to detect traumatic arthrotomies and identify periarticular wounds not requiring surgical intervention: an improvement over the saline load test. Journal of orthopaedic trauma. 2013;27(9):498-504. doi:10.1097/BOT.0b013e31828219bc
Metzger P, Carney J, Kuhn K, Booher K, Mazurek M. Sensitivity of the saline load test with and without methylene blue dye in the diagnosis of artificial traumatic knee arthrotomies. Journal of orthopaedic trauma. 2012;26(6):347-349. doi:10.1097/BOT.0b013e3182255167
Nord RM, Quach T, Walsh M, Pereira D, Tejwani NC. Detection of traumatic arthrotomy of the knee using the saline solution load test. The journal of bone and joint surgery american volume. 2009;91(1):66-70. doi:10.2106/JBJS.G.01682
Timothy D. Roberts. Traumatic arthrotomy with pneumarthrosis on plain radiograph of the knee. Western journal of emergency medicine. 2016;17(2):184-185. doi:10.5811/westjem.2015.12.29317
Expert Commentary
What a great review of traumatic arthrotomy! You now have a concise reference that teaches you everything you would probably ever need to know about this tricky diagnosis! These injuries are so uncommon that the first hurdle to overcome is actually considering the diagnosis. If you don’t consider it, then you hopefully just get lucky by a diagnostic x-ray that was ordered for other reasons!
Physical exam and exploration is indeed important but has limitations and does not rule out the diagnosis if the suspicion is high enough. The tract may be small, jagged, or there may be soft tissue destruction that limits your visualization. Be sure to inspect the wound while passively ranging the joint in question since it is often unclear the precise position of the joint (fully flexed, fully extended, or somewhere in between) when the wound occurred. This may bring the wound tract into your field of view. Ideally your exploration should be in a bloodless, painless field and documented as such.
While x-rays lack sensitivity, they are a worthwhile starting point since they are less expensive, noninvasive, readily available, and you can stop if they are positive. X-rays may also better define the extent and trajectory of the wound tract which my either heighten your suspicion or provide reassurance that the trajectory was away from the joint.
If the diagnosis is still in question, I prefer CT in most scenarios. It provides additional information about any associated fractures. CT is painless. Intra-articular air is very easy to see on CT. The downside is increased cost. Saline load testing seems to have more room for error. The joint must be properly entered. Enough fluid must be injected to fill the joint enough to cause visible extravasation. And the diagnosis can still be missed if it is forgotten to range the joint during the SLT. It is also quite painful. Consider all the patients you see who present with a painful joint effusion that has gradually accumulated. In the SLT you are giving the patient a sudden acute joint effusion. Ouch! So just be thoughtful about the route you choose to go.
Matthew Levine, MD
Associate Professor
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kermani, P. Herndon, A. (2022, Apr 25). Traumatic Arthrotomy. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/traumatic-arthrotomy
Other Posts You May Enjoy
C-Spine Intubation
Written by: Daniel Levine, MD (NUEM ‘24) Edited by: Zach Schmitz (NUEM ‘21)
Expert Commentary by: Matt Levine, MD
The “Evidence” Behind Manual In-Line Stabilization During Intubation of Trauma Patients
Background
Even in the absence of frank head and neck trauma that may cause bleeding or distortions in usual anatomy, trauma patients present challenging airways because of cervical spine precautions. Standard-of-care technique according to EAST (Eastern Association for the Surgery of Trauma), West (Western Trauma Association), and ATLS (Advanced Trauma Life Support) guidelines for intubating acute trauma patients with known or potential cervical spine injury involves manual in-line stabilization (MILS). (1,2) This is a two-person technique whereby one provider performs laryngoscopy while another holds the patient’s neck in place. The two most common techniques for this procedure are depicted below, one in which the stabilizer crouches down at the head of the bed (A), and the other where the stabilizer approaches from the side of the bed (B). (3)
(photo from Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition) (3)
Evidence
Like many practices in medicine, MILS has never been studied in randomized controlled trials, and the practice stems more from weak data and expert opinion. (4) The practice of spinal stabilization began during the 1970s after a retrospective review published in 1979 of 300 patients with acute cervical injuries who presented to Johns Hopkins hospital between 1950 and 1972. Although the main focus was on the effects of laminectomy and steroids, the review also found that 11 of the 300 patients developed neurologic deficits after reaching the hospital. Of the 11 patients, 7 developed these deficits “after neck immobilization was not provided”, with no clear comment as to whether immobilization was not provided during intubation or during some other process of the patient’s care. (5) These observations led to concerns that mobilization of the neck during intubation may worsen spinal cord injury, so manual in-line stabilization became standard of care in the 1980s.
Existing data for spinal stabilization comes from trials of cadaveric models, case series, and uninjured patients. Data from cadavers with post-mortem surgically created cervical spine injuries have shown mixed results on the effects of the amount of measured movement at the injured site with versus without MILS. For example, a 1993 study by Donaldson et al. found higher degrees of subluxation and angulation at C5-C6 during orotracheal intubation without MILS compared to with stabilization in five cadaveric specimens with injuries created in that area. (6) On the other hand, a 2001 Lennarson et al. study on cadavers found MILS significantly increased subluxation in C4-C5 during the same movements. (7) While it is somewhat counterintuitive that performing MILS might be associated with increased cervical motion, this may be explained by the laryngoscopist’s need to apply greater force with the laryngoscope in order to obtain an adequate view. This is what Santoni et al. (2009) found in a matched control study of 9 patients undergoing elective surgery. The patients in this study underwent two sequential laryngoscopies and oral intubations with a Macintosh 3 blade. Pressure transducers attached to the end of the blades detected higher maximum pressures at best glottic view with MILS compared to without. (8)
What is more clear in the literature on MILS than its effect on cervical motion is that it impairs glottic visualization and subsequent first pass intubation success. In the aforementioned Donaldson study on cadavers, MILS was shown to have a negative impact on Cormack-Lehane (CL) grade. (6) Similarly, in the aforementioned Santoni et al. study of 9 patients who underwent two sequential intubations with and without MILS, glottic visualization was worse in 6 patients with MILS, and intubation failure occurred in 2 of these 6 patients compared to no intubation failures among these patients when the intubation was performed without MILS. Thiboutot et al. (2008) performed a randomized controlled trial that further demonstrated this effect. In their study, 200 elective surgical patients were randomized to receive MILS or no MILS, and the primary endpoint was rate of failed intubation at 30 seconds with a Mac 3 blade. The rate of failed intubation was half in the MILS group (50%, 47/94), significantly higher compared to the control group (5.7%, 6/105). When they released manual in-line stabilization, they were able to intubate all patients. Secondary outcomes of rate of CL grade 3-4 as well as mean latency to successful intubation were also both significantly higher in the MILS group. (9) Additionally, these data were from patients undergoing elective surgery being intubated in the controlled OR setting by anesthesiologists. It is likely that the rate of failed intubation would be even higher in the chaotic emergency department environment with an acutely injured trauma patient. While 30 seconds is a somewhat arbitrary cutoff for a failed intubation, and it is quite possible many of the patients in the MILS group who “failed” may have been successfully intubated if a longer cut-off time were chosen, hypoxia caused by failed or delayed intubation is associated with poor outcome in central nervous system injury. (10)
Conclusion
In an ideal world, a large-scale randomized controlled trial of trauma patients studying the effects of MILS on mortality and important functional neurologic outcomes would help elucidate the utility of this commonly accepted practice. However realistically, completing such a study has significant obstacles. Cervical spine injuries are relatively rare (4% of trauma injured patients)4 and only a small fraction of those cases involve unstable injuries with potentially salvageable cord function. Thus, a study with sufficient power to detect any meaningful difference in outcomes would take many thousands of patients, many trauma centers, and many years to complete. Perhaps an even larger hurdle is the ethical and medicolegal hurdle of randomizing patients to not getting MILS and possibly putting them at risk of quadriplegia. (4) So what’s a clinician to do when faced with the common scenario of having to intubate a trauma patient? I personally like the approach that Dr. Reuben Strayer discusses in his video “Advanced Airway Management for the Emergency Physician” (link below). (11) To summarize his strategy:
*The exception: in the rare situation where the patient has a highly suspected (e.g. obvious bony deformity, focal neurologic deficit) or known cervical spine injury, Dr. Strayer recommends lowering the threshold to perform a cricothyroidotomy. Additionally, he recommends considering an awake intubation approach in these patients.
Another consideration is intubating using a hyper-angulated video GlideScope, which has been shown to have improved CL views and high rates of intubation success in c-spine immobilized patients. (12) That said, occasionally equipment availability or a bloody airway may preclude the use of video laryngoscopy in the trauma setting.
References
Mayglothling J, Duane TM, Gibbs M, McCunn M, Legome E, Eastman AL, Whelan J, Shah KH; Eastern Association for the Surgery of Trauma. Emergency tracheal intubation immediately following traumatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012 Nov;73(5 Suppl 4).
Brown CVR, Inaba K, Shatz DV, Moore EE, Ciesla D, Sava JA, Alam HB, Brasel K, Vercruysse G, Sperry JL, Rizzo AG, Martin M. Western Trauma Association critical decisions in trauma: airway management in adult trauma patients. Trauma Surg Acute Care Open. 2020 Oct 9;5(1)
Leonard, J et al. "Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition." Chapter 24: Cervical Spine Injury. https://doctorlib.info/pediatric/schafermeyers-pediatric-emergency-medicine/24.html, accessed 5/7/21.
Manoach S, Paladino L. Manual in-line stabilization for acute airway management of suspected cervical spine injury: historical review and current questions. Ann Emerg Med. 2007 Sep;50(3):236-45.
Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119- 1142.
Donaldson WF 3rd, Towers JD, Doctor A, et al. A methodology to evaluate motion of the unstable spine during intubation techniques. Spine. 1993;18:2020-2023
Lennarson PJ, Smith DW, Sawin PD, Todd MM, Sato Y, Traynelis VC. Cervical spinal motion during intubation: efficacy of stabilization maneuvers in the setting of complete segmental instability. J Neurosurg. 2001 Apr;94(2 Suppl):265-70.
Santoni BG, Hindman BJ, Puttlitz CM, Weeks JB, Johnson N, Maktabi MA, Todd MM. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009 Jan;110(1):24-31.
Thiboutot, F et al. Effect of manual in-line stabilization of the C-spine on the rate of difficult orotracheal intubation by direct laryngoscopy; a randomized controlled trial. Can J Anaesth. 2009 Jun;56(6):412-8.
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993 Feb;34(2):216-22.
“Advanced Airway Management for the Emergency Physician”, uploaded by Reuben Strayer, https://vimeo.com/12440392
Bathory I, Frascarolo P, Kern C, Schoettker P. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilisation by a semi-rigid collar. Anaesthesia. 2009 Dec;64(12):1337-41.
Expert Commentary
So once again a review of a significant body of literature leaves a clinical question unanswered, leaving the practitioner to either follow dogma or make one’s own conclusions. Like most of our medical decision making, this is a risk/benefit analysis. So let’s go through the process.
Some background context to keep in mind:
Most cervical spine injury occurs from the initial traumatic event (primary neurologic injury). Secondary neurologic injury is a cascade of events at the cellular level that worsen primary injury and is exacerbated by hypoxia and hypercarbia, which are frequent events in difficult/prolonged intubations. These must be minimized when the brain or c spine are injured!
The movements of the cervical spine that occur during ED care pale in magnitude to the cervical spine motion that caused the primary injury to occur. These likely contribute less to neurologic outcome than secondary neurologic injury from other events during ED care like hypotension, hypoxia, and hypocarbia.
It’s too difficult to intubate with a collar on. It must be carefully and temporarily removed. As Dr. Levine taught us, MILS impairs glottic visualization and first pass intubation success. Dr. Levine also taught us that we don’t know whether the injured cervical spine actually moves less or more with MILS during intubation attempts.
The synthesis:
These factors all lead me to agree with Dr. Strayer’s approach. It is reasonable to minimize cervical spine motion as much as possible, but not at the expense of adequate glottic visualization. Maybe MILS helps minimize motion during intubation. But abandon MILS when glottic visualization is suboptimal because MILS can be contributing to this, leading to hypoxia, hypercarbia, and secondary neurologic injury. Practice MILS only until it is possibly prolonging airway success, because now it is more likely to be harming than helping.
Even more future questions remain. Much of the prior literature is based on use of traditional orotracheal intubation techniques. How much of that knowledge applies to the now widespread use of fiberoptic video intubations (i.e. Glidescope), which may have better first pass success rates and less neck motion? Do we even need to perform MILS for these intubations? Or can we reliably rapidly intubate with MILS and the Glidecope – so we can have our cake and eat it too?
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Levine, D. Schmitz, Z. (2021, Oct 18). C-Spine. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/cervical-spine-intubation
Other Posts You May Enjoy
Managing Minor Thermal Burns in the ED
Written by: Mitch Blenden, MD (NUEM ‘24) Edited by: Vytas Karalius, MD, MPH, MA (NUEM ‘22) Expert Commentary by: Matt Levine, MD
Expert Commentary
Dr. Blenden and Dr. Karalius provided an excellent handy, high-yield, quick reference of thermal burn considerations in the ED. There are some nuances of thermal burn care that I’d like to provide further commentary:
A pitfall is underestimating the severity of the burn when the patient presents within a few hours of the event. Burn appearance evolves over 24-48 hours. What initially appears as erythematous skin can be covered in bullae the next day. Consider a repeat examination in 24-48 hours, or at least discuss with the patient the possibility that this may occur and what to do if it does. Otherwise, if you initially diagnosed the patient with superficial burns and provided only instructions for superficial burns, which require little treatment or follow-up, the patient can be set up for a worse outcome when these burns subsequently declare themselves to be partial thickness.
For years, most non-facial burns were sent home with instructions to use silver sulfadiazine (AKA Silvadene) cream. This would require teaching of how to apply and remove it. The cream needs to be removed daily before applying a new coat (I always sent the patient home with tongue blades to scrape it off). The benefits of this are that it debrides some nonviable tissue when the cream is removed and provides a moist antimicrobial barrier. The down sides are that removal can be painful and some patients have difficulty performing this procedure, which requires teaching. Silver sulfadiazine can also cause skin staining. There is scant evidence recommending one topical antimicrobial over another. For these reasons, practice (including mine) has evolved in many places to simply prescribe whatever antibiotic ointment is on hand for ease of use and less painful and technically challenging application.
Another controversy is whether to debride blisters and bullae or leave them intact. This is another area without definitive evidence and practice is often guided by gestalt, local custom, or prior teachings. On one hand, intact bullae can be thought of as “sterile” coverings and may be less painful than dermal layers exposed to air and friction. On the other hand, when bullae rupture, the patient is left with dead skin which can be a nidus for infection. My practice has been to leave small blisters intact and debride large bullae if it seems like they will soon rupture and leave the patient with hanging skin fragments. If the patient has reliable follow up burn care then I may choose a less aggressive approach in debriding. Other clinicians are likely to give alternate approaches so ask your attendings what they do in these scenarios so you can develop a practice pattern that makes sense to you.
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Blenden, M. Karalius, V. (2021, Oct 18). Managing Minor Thermal Burns in the ED. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/managing-minor-thermal-burns
Other Posts You May Enjoy
SonoPro Tips and Tricks for Pneumothroax
Written by: Morgan McCarthy, MD (NUEM ‘24) Edited by: Jon Hung, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Did you know that Lung Ultrasound (LUS) has a higher sensitivity than the traditional upright anteroposterior chest X-ray for the detection of a pneumothorax? (LUS has a reported 90.9 for sensitivity and 98.2 for specificity. CXR were 50.2 for sensitivity and 99.4 for specificity). Busy trauma bay? Ultrasound is faster than calling for X-ray. Critically ill patient? Small pneumothoraces are less likely to be missed with ultrasound. To take your Sono Skills to the next level, read on:
Beyond the classic trauma patient during your E-Fast Exam, who else does the Sono-Pros scan?
Primary spontaneous pneumothorax: the classic scenario is a tall, young adult, with symptoms such as breathlessness, along with potentially those with risk factors of pneumothoraxes such as smoking, male sex, family history of pneumothorax
Secondary spontaneous pneumothorax: those with underlying lung disease including but not limited to COPD, tuberculosis, necrotizing pneumonia, pneumonocystis carini, lung cancer, sarcoma involving the lung, sarcoidosis, endometriosis, cystic fibrosis, acute severe asthma, idiopathic pulmonary fibrosis
Of course, traumatic pneumothorax, especially in penetrating trauma or blunt trauma with broken ribs
Don’t forget iatrogenic causes of pneumothorax including transthoracic needle aspiration, subclavian vessel puncture, thoracentesis, pleural biopsy, and mechanical ventilation
SonoPro Tips - How to scan like a Pro
The key is to have the patient completely supine - air rises! - with the probe in the anterior field in sagittal orientation pointing towards the patient's head.
It is commonly taught to start at the second intercostal space, midclavicular line, and scan down a few lung spaces to at least the 4th intercostal space, however, keep in mind some studies show that trauma supine trauma patients had pneumothoraces seen more commonly in the 5-8 rib spaces.
Important Landmarks
Green = Subcutaneous tissue. Red = Pleural space. Blue = A - lines.
4. Look for lung sliding, improve your image by turning down gain and decrease depth to have lung sliding become clearer
What to Look For:
To Rule-Out a pneumothorax
Lung Sliding - Lung sliding has a negative predictive value of 100% for ruling out a pneumothorax, however only at that interspace
Additional Findings: B-lines and Z lines also help to rule out pneumothorax!
2. To Rule-In a pneumothorax
Lung point - the interface between where lung sliding is happening and where the absence of lung sliding is happening has been shown to have 100% specificity for pneumothorax.
Keep in mind the border of where the heart and lung come in contact and the border where the diaphragm and lung come in contact can cause a false lung point.
The lung point may be hard to find in a larger pneumothorax, and impossible to find in a completely collapsed lung.
3. Next turn on M-mode:
Sandy Beach Shore = Lung sliding (left). Barcode Sign = No lung sliding (right)
What to do next:
Lung sliding = sensitive, Lung point = specific
If you see lung sliding, there is no pneumothorax
If you do not see lung sliding it does not rule in a pneumothorax -> look for a lung point, the interface between where lung sliding is happening and where the absence of lung sliding is happening to rule it in
Always keep in mind other causes that result in lack of lung sliding before management decisions take place!: atelectasis, main-stem intubation, adhesions, contusions, and arrest or apnea. Check out this great table from 5 - Min Sono.
4. If your patient is apneic or has a mainstem intubation look for lung pulse, when the heart beats if the parietal and visceral pleura are touching (no pneumothorax) it will show a pulse at the interfaces of the pleura
5. Sub-Q emphysema - Always look for E - lines. When there is subcutaneous air above the pleural line it creates a false pleural line above the actual pleural. You may also see B-lines obscuring the actual pleural line. This is most likely subcutaneous air and you can not interpret it for a pneumothorax.
SonoPro Tips - Where to Learn More
American College of Emergency Physicians. Emergency ultrasound imaging criteria compendium. Ann Emerg Med. 2006;48(4):487-510.
Ma, John, et al. Ma and Mateer's Emergency Ultrasound. McGraw-Hill Education, 2020.
Macias, Micheal. TPA, The Pocus Atlas.
Availa, Jacob. 5 minute Sono.
Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708.
Expert Commentary
Morgan went “beyond lung sliding” and dove deep into how to increase your sensitivity & specificity for PTX with POCUS. Supine is ideal to make PTX visible against the anterior chest wall, but if the patient cannot tolerate lying flat, look at the apical pleural superior to the clavicles. First, identify the true pleural line--it should be the bright line just deep to the ribs in your view. SQ emphysema may obscure the view or even mimic the pleura, although its outline is usually more hazy & irregular, a little pressure helps to move the SQ air out of the way can be helpful. Sliding? Great, PTX ruled out. But absent sliding does not automatically mean PTX. Make sure there is no B-line or “lung pulse”, as sometimes pleural adhesion or poor ventilation can cause absent sliding too. Most of the time you don’t need M-mode unless the movement is very subtle and you want to be extra sure. The lung point is pathognomonic for PTX, but don’t waste time digging around for it if the patient is unstable with a good clinical story for PTX > decompress instead!
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] McCarthy, M. Hung J. (2021 Sept 20). SonoPro Tips and Tricks for Pneumothorax. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-pneumothorax
Other Posts You May Enjoy
Hanging Injuries
Written by: Vytas Karalius, MD, MPH (NUEM ‘22) Edited by: Nery Porras, MD (NUEM ‘21) Expert Commentary by: Kevin Emmerich, MD, MS
Today’s post was inspired by the near-hanging of young gentleman who ended up passing away due to complications related to his near-hanging. His parents decided to donate his organs to Gift of Hope, allowing the passing of his life to extend the lives of others. While we hope to never see cases like these, they are an inevitable part of our job as emergency medicine physicians. As with most rare and complex pathology, preparation and knowledge can help us with the management of these cases when things often get chaotic. Lastly, as emergency medicine physicians who see the sequelae of mental illness daily in their EDs, I encourage us all to advocate for better funding and access to mental health care in the United States.
Hanging Injury
Terms/Classification [1]
“Hanging” is used to describe a death from a form of strangulation that involves hanging from the neck.
“Near-hanging” is a term for patients who have survived an attempted hanging (or at least long enough to reach the hospital).
“Complete hanging” defines when a patient’s legs are fully suspended off the ground and the patient's bodyweight is fully suspended by the neck.
“Incomplete hanging” defines when some part of the patient’s body is still on the ground and the body's full weight is not suspended off the ground.
“Judicial hanging” classically refers to victims who fell at least the height of their body.
Epidemiology:
Hanging is the 2nd most common form of successful suicide in the US after firearms
Accounts for 23% of >34,500 suicides in 2007
In the jail system, hanging is the most common form of successful suicide
Increasing incidence in US
Risk Factors: male, aged 15-44 years, history of drug or alcohol abuse, history of psychiatric illness
Pathophysiology of Injury:
Spine/Spinal Cord:
When the drop is greater than or equal to the height of the victim, as in a judicial hanging, there will almost always be cervical spine injury.
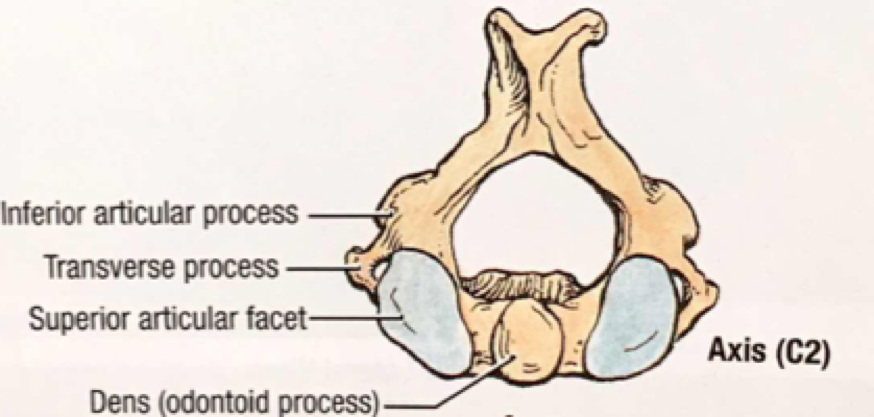
The head hyperextends, leading to fracture of the upper cervical spine ("hangman's fracture” of C2) and transection of the spinal cord.
Cervical injuries are in non-judicial hangings are rare. [2] One retrospective case review of near-hangings over a 10-year period found the incidence of cervical spine fracture to be as low as 5%. [3]
Vascular:
The major pathologic mechanism of death in hanging/strangulation is neck vessel occlusion, not airway obstruction. [1,4]
Death ultimately results from cerebral hypoxia and global ischemia.
There are two mechanisms by which this happens:
Venous: The most implicated cause of death is actually venous obstruction. Jugular veins are superficial and easily compressible. Obstruction of venous outflow from the brain leads to stagnant hypoxia and loss of consciousness in as little as 15 seconds.
Arterial: The risk of damage to the major arterial blood flow to the brain (such as carotid artery dissection) is rare, but should suspected in patients. [4]
Cardiac:
Carotid body reflex-mediated cardiac dysrhythmias are reported, and likely a minor mechanism of death.
Pulmonary:
Airway compromise plays less of a role in the immediate death of complete hanging/strangulation. However, it is a major cause of delayed mortality in near-hanging victims. [1,4]
Significant pulmonary edema occurs through two mechanisms:
Neurogenic: centrally mediated, massive sympathetic discharge; often in association with serious brain injury and a poor prognostic implication.
Post-obstructive: strangulation causes marked negative intrapleural pressure, generated by forceful inspiratory effort against extra-thoracic obstruction; when the obstruction is removed, there is a rapid onset pulmonary edema leading to ARDS.
Aspiration pneumonia later sequela of near-hanging injury.
Airway edema from mechanical trauma to the airway, which can make intubation difficult.
Tracheal stenosis can develop later in the hospital course.
Other Injuries:
Hyoid bone fracture
Cricoid or thyroid cartilage injury [5]
Physical Examination:
"Ligature marks" or abrasions, lacerations, contusions, bruising, edema of the neck
Tardieu spots of the eyes
Severe pain on gentle palpation of the larynx (laryngeal fracture)
Respiratory signs: cough, stridor, dysphonia/muffled voice, aphonia
Varying levels of respiratory distress
Hypoxia
Mental status changes
Early Management/Stabilization:
ABCs as always
Early endotracheal intubation may become necessary with little warning.
Patients who are unconscious or have symptoms such as odynophagia, hoarseness, neurologic changes, or dyspnea require aggressive airway management.
If ETI unsuccessful, consider cricothyroidotomy; if unsuccessful, percutaneous trans-laryngeal ventilation may be used temporarily.
Judicious and cautious fluid resuscitation - avoid large fluid volume resuscitation and consider early pressors, as fluids increases the risk/severity of ARDS and cerebral edema.
Monitor for cardiac arrhythmias.
The altered/comatose patient should be assumed to have cerebral edema with elevated ICP.
Imaging/Further Testing:
Chest radiograph
CT brain
CT C-spine
CTA head/neck
Can consider soft-tissue neck x-ray, if CT not immediately available
Further Management:
In patients with signs of hanging/strangulation, there should be a low threshold to obtain diagnostic imaging/testing as discussed above.
Expect pulmonary complications early.
They are a major cause of delayed mortality in near-hanging victims, as stated above.
Early intubation and airway management are important.
Non-intubated patients with pulmonary edema may benefit from positive end-expiratory pressure ventilation.
Patients with symptoms of laryngeal or tracheal injury (e.g. dyspnea, dysphonia, aphonia, or odynophagia), should undergo laryngobronchoscopy with ENT. [4,6]
Tracheal stenosis has been reported during the hospital course. Address cerebral edema from anoxic brain injury, using strategies to reduce intracranial pressure or seizure prophylaxis. [4]
Address vascular complications seen on CTA and coordinate intervention with the appropriate specialty at your institution.
Therapeutic Hypothermia
There is some evidence for therapeutic hypothermia in those with cardiac arrest from hanging injury [7,8] and those who are comatose from hanging injury. [9-11] While the evidence is weak, in the absence of better evidence, it is reasonable to consider hypothermia treatment in all comatose near-hanging victims. [1,12,13]
When suicide is suspected, evaluate patients for other methods of self-harm (e.g. wrist lacerations, self-stabbing, ingestions). It is also important to consider drug and alcohol intoxication. [4]
Disposition:
Admit critically ill patients to the appropriate ICU-level care.
Admit patients with abnormal radiologic or endoscopic imaging to the appropriate service and level of care.
Even if the initial presentation is clinically benign, all near-hanging victims should be observed for 24 hours, given the high risk of delayed neurologic, airway and pulmonary complications. [14]
Observe asymptomatic patients with normal imaging.
Psychiatry/Crisis Team consult on all suspected intentional cases.
Emphasize strict return precautions as well as education about possible delayed respiratory and neurologic dysfunction when discharging patients.
Some patients may require transfer to a trauma center if the required services are not available at the initial receiving facility. [1]
Prognostication:
GCS 3/GCS 3T is a predictor of very poor outcome, [15-19] but there is mixed evidence on the GCS as a predictor of outcomes in GCS scores greater than 3, especially with regard to neurologic intactness. [3,19]
Recovery of patients with neurology symptoms is unpredictable. [4]
Patients presenting with cardiac arrest have a very poor prognosis, and might be the strongest predictor of poor prognosis. [4,8,16,18,20]
Other predictors of poor clinical outcome include:
Anoxic brain injury or cerebral edema on head CT [3,19]
Prolonged hanging time [18]
Cardiopulmonary arrest [8,11,19]
Cervical spine injury
Hypotension on arrival
Expert Commentary
We’ve all certainly been involved with a patient with reported hanging injury at some point in our time in the ED. They are usually unimpressive if a person does it as more of a gesture rather than a true suicide attempt. When they are unfortunately done “correctly,” they usually result in a trip to the morgue instead of the ED. When the swiss cheese holes align and a true hanging attempt results in a serious but not fatal presentation, things can get quite hairy. I’ve been a part of one such case, and will never forget it. Here are my two cents.
Airway
This should undoubtedly be treated as a predicted difficult airway, not only due to likely cervical spine trauma, but also possibly due to airway edema. Get your ducks in a row for this unless this patient is crashing in front of you. Get your consultants/help (if available), preoxygenate, airway adjuncts open and ready, backup airway supplies if your first plan fails. Most importantly, have a plan and discuss this with your team beforehand. Don’t be afraid to take an awake look with a hyperangulated video laryngoscope, especially if this patient presents with stridor. Ketamine can be your friend here. This should be an airway that you do not undertake without a scalpel, finger, and bougie ready just in case. I like to draw a line on the patient’s skin overlying the cricothyroid membrane beforehand.
Trauma
Self explanatory, but don’t be stingy here. Light this patient up from head to pelvis, including the neck angiogram. Document a repeat neuro exam every time you move this patient.
Overdose/psych
Don’t forget your Tylenol and salicylate levels, EKG in this suicide attempt. If you feel the need to add the useless urine drug screen, I suppose this is fine as well.
Kevin Emmerich, MD, MS
Emergency Medicine Physician
Methodist Hospital
Gary, Indiana
How To Cite This Post:
[Peer-Reviewed, Web Publication] Karalius, V. Porras, N. (2021, Aug 9). Hanging Injuries. [NUEM Blog. Expert Commentary by Emmerich, K]. Retrieved from http://www.nuemblog.com/blog/hanging-emergencies
Other Posts You May Enjoy
References
1. Walls RM, Hockberger RS, Gausche-Hill M. Rosen's emergency medicine : concepts and clinical practice. Ninth edition. ed. Philadelphia, PA: Elsevier; 2018.
2. Aufderheide TP, Aprahamian C, Mateer JR, et al. Emergency airway management in hanging victims. Ann Emerg Med. 1994;24(5):879-884.
3. Salim A, Martin M, Sangthong B, Brown C, Rhee P, Demetriades D. Near-hanging injuries: a 10-year experience. Injury. 2006;37(5):435-439.
4. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli's emergency medicine: a comprehensive study guide. 9th. ed. New York: McGraw-Hill Education; 2019.
5. Tugaleva E, Gorassini DR, Shkrum MJ. Retrospective Analysis of Hanging Deaths in Ontario. J Forensic Sci. 2016;61(6):1498-1507.
6. Hackett AM, Kitsko DJ. Evaluation and management of pediatric near-hanging injury. Int J Pediatr Otorhinolaryngol. 2013;77(11):1899-1901.
7. Hsu CH, Haac B, McQuillan KA, Tisherman SA, Scalea TM, Stein DM. Outcome of suicidal hanging patients and the role of targeted temperature management in hanging-induced cardiac arrest. J Trauma Acute Care Surg. 2017;82(2):387-391.
8. Kim MJ, Yoon YS, Park JM, et al. Neurologic outcome of comatose survivors after hanging: a retrospective multicenter study. Am J Emerg Med. 2016;34(8):1467-1472.
9. Jehle D, Meyer M, Gemme S. Beneficial response to mild therapeutic hypothermia for comatose survivors of near-hanging. Am J Emerg Med. 2010;28(3):390.e391-393.
10. Lee BK, Jeung KW, Lee HY, Lim JH. Outcomes of therapeutic hypothermia in unconscious patients after near-hanging. Emerg Med J. 2012;29(9):748-752.
11. Hsu CH, Haac BE, Drake M, et al. EAST Multicenter Trial on targeted temperature management for hanging-induced cardiac arrest. J Trauma Acute Care Surg. 2018;85(1):37-47.
12. Borgquist O, Friberg H. Therapeutic hypothermia for comatose survivors after near-hanging-a retrospective analysis. Resuscitation. 2009;80(2):210-212.
13. Sadaka F, Wood MP, Cox M. Therapeutic hypothermia for a comatose survivor of near-hanging. Am J Emerg Med. 2012;30(1):251.e251-252.
14. McHugh TP, Stout M. Near-hanging injury. Ann Emerg Med. 1983;12(12):774-776.
15. Kao CL, Hsu IL. Predictors of functional outcome after hanging injury. Chin J Traumatol. 2018;21(2):84-87.
16. La Count S, Lovett ME, Zhao S, et al. Factors Associated With Poor Outcome in Pediatric Near-Hanging Injuries. J Emerg Med. 2019;57(1):21-28.
17. Martin MJ, Weng J, Demetriades D, Salim A. Patterns of injury and functional outcome after hanging: analysis of the National Trauma Data Bank. Am J Surg. 2005;190(6):836-840.
18. Matsuyama T, Okuchi K, Seki T, Murao Y. Prognostic factors in hanging injuries. Am J Emerg Med. 2004;22(3):207-210.
19. Nichols SD, McCarthy MC, Ekeh AP, Woods RJ, Walusimbi MS, Saxe JM. Outcome of cervical near-hanging injuries. J Trauma. 2009;66(1):174-178.
20. Gantois G, Parmentier-Decrucq E, Duburcq T, Favory R, Mathieu D, Poissy J. Prognosis at 6 and 12months after self-attempted hanging. Am J Emerg Med. 2017;35(11):1672-1676.
Scalpel Finger Bougie
Written by: Em Wessling, MD (NUEM ‘22) Edited by: Therese Whipple (NUEM ‘20) Expert Commentary by: Joseph Posluszny, MD
Expert Commentary
Establishing an airway via a cricothyroidotomy is a stressful and tense experience. In almost all of these cases, experienced airway staff have already attempted advanced airway maneuvers in patients typically at high risk for inability to intubate. As the oxygen saturation drops and the patient becomes unstable, the most adept proceduralist present (whether emergency department physicians or surgeons) are asked to step in to secure a surgical airway.
The scalpel-finger-bougie technique is one proven and reliable method to secure a surgical airway via a cricothyroidotomy. Some additions to the technique described above are:
Use a vertical incision through the skin and soft tissues. If you are too superior or inferior with your initial incision, then this incision can be easily extended as needed. A horizontal incision commits you to that cranial-caudal level. It is often more of a struggle to identify the cranial-caudal orientation of the cricothyroid membrane rather than the medial-lateral orientation.
In a patient with a stable and flexible neck, retract the neck via cranial pressure on the chin to bring the neck structures better into your working field. Insert a shoulder roll if available (unlikely) to augment this positioning.
After the tube is advanced, listen for bilateral breath sounds. It is common, in this adrenaline fueled procedure, to advance the endotracheal tube too far, leading to a right main stem intubation. This can limit your ventilation and oxygenation and can lead to confusion about the airway placement in the neck. If there are no left lung field breath sounds, then pull the tube back until bilateral breath sounds are confirmed with auscultation.
Always verify tube placement with capnography.
Persistent, moderate volume bleeding is often from injury to the anterior jugular vein. Gentle, directed pressure on the area can control this bleeding while the patient is being transported to the operating room for a more definitive airway.
Joseph Posluszny, MD
Assistant Professor of Surgery (Trauma and Critical Care)
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Wessling, E. Whipple, T. (2021, Aug 2). Scalpel Finger Bougie. [NUEM Blog. Expert Commentary by Posluszny, J]. Retrieved from http://www.nuemblog.com/blog/scalpel-finger-bougie
Other Posts You May Enjoy
Non-Accidental Trauma - A Can’t Miss Diagnosis
Written by: Dana Loke, MD (NUEM PGY-4) Edited by: Ashley Amick, MD (NUEM ‘18) Expert commentary by: Lauren Riney, DO
Introduction
Non-accidental trauma (NAT) is a leading cause of pediatric traumatic injury and death. In 2014 alone, there were 1546 reported deaths from NAT and 3.6 million child abuse referrals submitted to Child Protective Services (CPS). [1] NAT is most commonly encountered in young children, but can occur at any age. The classic signs and symptoms of NAT will be reviewed here, but it is important to realize that occult injury is common. Compared with accidental pediatric trauma, patients with NAT have been shown to have higher injury severity scores, rates of intensive care unit admission, and mortality. Furthermore, the diagnosis of NAT is delayed in 20% of cases, increasing the risk of poor outcomes.[2] Therefore, the Emergency Physician (EP) must maintain a high index of suspicion for NAT to prevent the grave consequences of missed diagnosis for the patient and any other children in the home.
Red Flags and Risk Factors
NAT is a frequently missed diagnosis, but there are some red flags and risk factors that should make the EP take pause and consider this diagnosis. Children at greatest risk are generally toddler and younger, and often come from dysfunctional family units. A recent study found that 97% of NAT cases have antecedent familial dysfunction, such as substance abuse (alcohol or drugs), psychiatric disorder, history of violence or incarceration, or child withdrawal. [3] Additionally, over 70% of reported NAT deaths in 2014 were in children under 3 years old. [1]
Red Flags
Injuries inconsistent with the caregiver’s history
Reported mechanism of injury is unexpected for the child’s developmental status (for instance, a 2 week old infant rolling off of a bed)
Delayed presentation
Risk Factors
Age under 5 account for 81.5% of cases; children under 1 are most vulnerable [3]
Prematurity
Multiple medical conditions
Young parent
Female parent (although males are more likely to inflict fatal NAT)
Poor social support
Unplanned or unwanted pregnancy
Poor prenatal care
Shorter birth intervals between children
Increased number of separations from the child in the first year
Abuser Characteristics
Poor self-esteem
Depression and suicide attempts
Life stressors
Personal history of being abused as a child
Exposure to foster care or abandonment as a child
Engagement in criminal activity or corporal punishment as a child
Many other suspected risk factors have been studied. There is no consensus regarding whether a particular race is at greatest risk for NAT however black children have a greater risk of mortality from NAT. [4] Similarly, there is no consensus regarding socioeconomic status as it relates to NAT risk, but studies have shown that incidence of non-accidental head trauma and its severity rise during times of economic recession. [4]
Presentation
Figure 1: Bruising patterns that suggest child abuse. [6]
Bruising
Bruising is the most common manifestation of NAT but has low specificity. In any child presenting with bruising, it is imperative to note the location, shape and pattern of the lesion and ensure this is clearly documented. Bruising located over soft tissue areas such as the cheeks, neck, genitals, buttocks, torso, and back, are more likely to represent NAT than bruises over bony prominences. [4] The shape of the bruise should be considered as well, since the bruise often reflects the shape of the causative object. Common objects used to inflict injury include belts, cords, shoes, kitchen utensils, hangers, and teeth. [4] Additionally, patterned bruises should raise suspicion for NAT since they generally do not occur with accidental trauma. Lastly, any bruising in non-mobile infants is suspicious for NAT as well. [5]
Figure 2: Forced immersion burn of buttocks with bilateral, symmetric leg involvement in a “stocking” pattern. [7]
Burns
Burns occur in 8-12% of NAT cases. [2] The most common types of burns from NAT are scald burns and thermal contact burns. Scald burns are the most common and typically occur from forced immersion in hot liquids, usually of the buttock, or in a stocking-and-glove distribution. Scald burns generally have sharp demarcation, uniform depth, and lack splash or drip marks that would be seen in an accidental immersion. Thermal burns occur from contact with hot objects, of which branding with metal implements or cigarettes is a common presentation. Concerning features of burns include:
Location on the hands (especially the dorsum), legs, feet, or buttocks
Patterned contact burns in the shape of an object (such as a fork, clothing iron, curling iron, or cigarette lighter)
Sharp stocking-and-glove pattern with sparing of the flexed protected areas (the classic forced immersion burn pattern)
Figure 3: Classic metaphyseal lesion. White arrows denote femoral metaphyseal separation and black arrow denotes a proximal tibial lesion or “bucket handle.” [1]
Fractures
There are various non-accidental fracture patterns, several with high specificity as described below:
Classic metaphyseal lesion (CML) – Also known as “bucket handle fractures” or “corner fractures,” these fractures are highly specific in children less than one year old. They result from a shearing force applied to a long bone, which causes avulsion of the metaphysis. These fractures are not associated with falls.
Multiple posterior and/or lateral rib fractures – These fractures also have a high correlation with NAT in children less than one year old. They arise from a specific mechanism – grasping the child around the torso and exerting a squeezing/compressive force. These fractures are more likely to affect the rib head and neck given the closer proximity to the transverse processes of the spine. NAT should especially be considered when healing fractures are found in a child without recent CPR.
Figure 4: Posterior and lateral rib fractures of differing ages indicative of NAT [4]
Clavicular fractures and spiral fractures of long bones in nonambulatory children
Multiple fractures, especially if in different stages of healing
Scapular fractures
Sternal fractures
Spinous process fractures
Of note, spiral fractures of long bones generally result from twisting injuries (indicating NAT), but can occur accidentally from falls in ambulatory children. Therefore, these fractures (especially if coupled with clavicular fractures) are more specific for NAT in younger patients, and the specificity decreases with advancing age. Other described non-accidental patterns to consider include epiphyseal separations, vertebral body fractures and separations, digital fractures, linear and complex skull fractures, and subperiosteal bone formation. These patterns have low to moderate specificity for NAT. [1]
Abusive Head Trauma
Abusive head trauma (AHT) is the most fatal form of non-accidental injury in children. In fact, about 80% of deaths from NAT are caused by AHT and only 15% of patients with AHT survive without any sequelae. [4] AHT is a spectrum of injuries including collisions with stationary objects, direct blows to the head, and a repetitive acceleration- deceleration injury, also known as “Shaken Baby Syndrome.” Infants are particularly vulnerable to traumatic brain injury from shaking due to the relative weight of the head compared to the body, coupled with weak neck musculature. [1] If AHT is suspected, a non-contrast head CT should be obtained even with a nonfocal neurologic examination, because occult intracranial injury is common. Make sure to use age-appropriate dose reduction to minimize radiation exposure and if the CT scan is normal, consider further work-up with an MRI.
Figure 5: Fundus of child with AHT with too-numerous-to-count retinal hemorrhages indicated by the black arrows. [8] The white arrow indicates small pre-retinal hemorrhages. The white arrowhead denotes hemorrhage extending into the peripheral retina. The black arrowhead denotes a healthy optic disc.
Ocular Manifestations
Although there are many ocular manifestations associated with non-accidental head injuries, retinal hemorrhages occur most often (about 60-85% of non-accidental head injuries). [4] Suspicion for NAT should be especially heightened when retinal hemorrhages are found in combination with signs of head trauma. Other ocular manifestations of NAT include periorbital hematoma, eyelid laceration, subconjunctival hemorrhage, subluxed or dislocated lens, cataracts, glaucoma, anterior chamber angle regression, iridiodialysis, retinal dialysis or detachment, intraocular hemorrhage, optic atrophy, or papilledema. [4]
Management and Disposition
All patients with suspected NAT should be admitted for protection and coordination of care even if they are clinically stable. Child Protective Services (CPS) must be notified, and engagement with the institutional social worker and child abuse team is recommended. It is important to note patients with NAT often have worse outcomes than other assault patients despite similar mechanisms of injury with intent to harm. [9] These patients often require close monitoring with Intensive Care Unit (ICU) resources. Patients with NAT should undergo a full skeletal survey as indicated in Figure 6 with additional imaging (CT, MRI) tailored to each patient. For instance, CT abdomen and pelvis should be obtained per general trauma guidelines, particularly if there is suspicion for solid organ or visceral injury.
Figure 6: Elements of the Skeletal Survey. Although a full skeletal survey is currently the standard of care for patients with NAT, there are ongoing research efforts to tailor X-ray imaging more specifically to each patient. [1]
Other diagnoses to consider in these patients include metabolic bone disease (such as rickets, Caffey disease, and osteogenesis imperfecta), blood dyscrasias, benign enlarged subarachnoid spaces (BESS), glutaric aciduria type 1 (which causes brain atrophy and subdural fluid collections). [1] However NAT is far more common than these diagnoses and carries significant morbidity and mortality when overlooked so should be considered and worked-up prior to these diagnoses.
Key Points
Pediatric NAT causes significant morbidity and mortality, and therefore EPs must maintain a high degree of suspicion for this diagnosis.
Red flags during evaluation include a changing or inconsistent history, injuries inconsistent with the history, an unexpected mechanism of injury based on the child’s developmental status, and delayed presentation despite significant injury.
Risk factors for NAT include children younger than school age (with children younger than 1 being most vulnerable), family dysfunction, prematurity, multiple medical conditions, young/female parent, poor social support, unplanned or unwanted pregnancy, poor prenatal care, numerous separations from the child in the first year of life, and history of psychiatric issues, stressors, criminal activity, or childhood abuse or abandonment in the abuser.
Although physical exam findings can be non-existent or non-specific, highly specific findings include bruising over soft tissue areas; bruises/burns that are patterned take the form of an object; any bruising in a non-mobile child; scald burns on the hands, legs, feet, or buttocks; and stocking-and-glove patterned burns.
Highly concerning fracture patterns include classic metaphyseal lesions (“bucket handle fractures” or “corner fractures”), multiple posterior and/or lateral rib fractures, clavicular or spiral long bone fractures in any nonambulatory child, multiple fractures, fractures in different stages of healing, scapular fractures, sternal fractures, and spinous process fractures.
There is a wide range of ocular manifestations in NAT but the most common manifestation is retinal hemorrhage(s).
AHT carries the highest mortality rate of all the injuries associated with NAT. Any suspicion for AHT warrants consideration of a non-contrast head CT.
Notify Child Protective Services (CPS) and admit these children for further NAT work-up including a full skeletal survey.
Expert Commentary
Excellent overview of NAT in the Emergency Department with emphasis on risk factors and manifestations. I want to add a few pearls about NAT and then will focus my commentary on NAT management in the ED as well as discussion with families, as this was recently a large quality improvement project in our pediatric tertiary care center.
Neglect is the most common form of child abuse accounting for about two-thirds of all forms of abuse and often accompanies other forms of abuse. (1) Neglect is involved in about 50% of all cases of fatal child abuse. (1) Among children less than 1 year of age, 25% of fractures are a result of abuse. (2) Consider two things: does the explanation the provider stated account for the fracture the child has sustained? Is the child developmentally capable of the action being described? After 2 years of age, the history and physical exam should determine the imaging required. Over 5 years of age, the yield of unsuspected fractures from a skeletal survey is only 9%, making this group more amenable to selective radiographic studies. (3)
Diagnosis of NAT in children remains a challenge due to provider bias, preconceptions, and failure to recognize the presentation as possible abuse. (4,5) As a result, these injuries may go undetected, leading to further injury prior to diagnosis. An estimated 25% of children ultimately diagnosed with NAT have a sentinel injury prior to their abuse diagnosis. (6,7) Of abused children with a previous sentinel injury, the most common were a bruise (80%), a torn frenulum (11%), or a fracture (7%). (8) A large retrospective chart review estimated 80% of deaths from unrecognized abusive head trauma may have been prevented by earlier detection of NAT. (6) The American Academy of Pediatrics (AAP) states that “ANY injury to a young, pre-ambulatory infant” suggests abuse. (9)
Figure 1: Standardized Physical Abuse Guideline.
At our institution, a team of pediatric emergency medicine physicians and child abuse pediatricians convened to develop and implement a standardized NAT guideline for providers in the ED when evaluating children with suspected NAT (Figure 1 Standardized Physical Abuse Guideline). This work stemmed from a chart review showing there was significant variability in the evaluation and management of children with concern for NAT in our Pediatric Emergency Department. The guideline was based on current peer reviewed literature as well as local expert consensus. It is divided into three separate age groups: < 6 months, 6-12 months, and >12-36 months. Age groups were determined based on risk of injury at different age levels in described literature, acquisition of milestones as age progresses, and increased ability for young children to show specific signs of injury with increasing age.
Lastly, the evaluation of NAT is stressful for both families and healthcare providers. The second page of our NAT guideline gives a sample script for EPs when discussing the non-accidental trauma evaluation for children. It states, “Any time a child comes to the hospital with this injury/these injuries, we evaluate for other injuries. Sometimes a child can have internal injuries such as fractures, head injury or abdominal injuries that we cannot see on the outside. Just like you, we want to make sure that your child is okay, so it is important to do this testing. We will also have our social worker come talk to you. This is a standard part of our evaluation. We are happy to answer any questions along the way”. It is important to acknowledge that this process is stressful, time consuming, and not comfortable for the child. Explaining each part of the process is important. Ensure that you use language that is non-accusatory. As EPs, we are not the ones to identify who the perpetrator is/was, but rather ensure the full NAT evaluation is completed and allow social work and/or Child Protective Services to determine further action.
Non-accidental trauma remains too prevalent in our country. Literature continues to show that unrecognized NAT leads to worse injuries and sometimes fatality. Continuing knowledge and education about injuries suspicious for NAT for EPs remains imperative. Standardized evaluations and real time order sets can increase appropriate management of NAT in the Emergency Department.
References:
Dubowitz H. Epidemiology of Child Neglect. CAN 2011, pp 28-34.
Kaczor K, Clyde Pierce M. Abusive Fractures. CAN 2011, pp 275-295.
Martich KV. Imaging of Skeletal Trauma in Abused Children. CAN 2011, pp 296-308.
Higginbotham N, Lawson KA, Gettig K, et al. Utility of a child abuse screening guideline in an urban pediatric emergency department. J Trauma Acute Care Surg. 2014;76(3):871-877.
Tiyyagura GK, Gawel M, Koziel JR, et al. Barriers and facilitators to detecting child abuse and neglect in general emergency departments. Annals of Emergency Medicine. 2015;66(5):447-454.
Jenny C, Hymel K, Ritzen A, et al. Analysis of missed cases of abusive cases of head trauma. JAMA. 1999;282:621-6.
Rangel EL, Cook BS, Bennett BL, et al. Eliminating disparity in evaluation for abuse in infants with head injury: use of a screening guideline. Journal of Pediatric Surgery. 2009; 44(6):1229-34.
Sheets LK, et al. Injuries in Infants Evaluated for Child Physical Abuse. Pediatrics. 2013, pp 701-707.
Christian CW, Committee on Child Abuse and Neglect. The evaluation of suspected child physical abuse. Pediatrics. 2015;135:e1337–e1354.
Lauren C. Riney, DO
Assistant Professor
Division of Emergency Medicine
UC Department of Pediatrics
How to Cite this Post
[Peer-Reviewed, Web Publication] Loke D, Amick A. (2019, Oct 7). Non-Accidental Trauma. [NUEM Blog. Expert Commentary by Riney C]. Retrieved from http://www.nuemblog.com/blog/nonaccidental-trauma.
Other Posts You Might Enjoy
References
Pfeifer, C.M., Hammer, M.R., Mangona, K.L., & Booth, T.N. (2017). Non-accidental trauma: the role of radiology. Emerg Radiol, 24, 207-213.
Kim, P.T. & Falcone, R.A. (2017). Non-accidental trauma in pediatric surgery. Surgical Clinics of North America, 97.1, 21-33.
Child maltreatment 2014. Report, Children’s Bureau. Washington, DC: U.S. Department of Health and Human Services; 2014. Available at: http://www.acf. hhs.gov/sites/default/files/cb/cm2014
Paul, A.R. & Adamo, M.A. (2014). Non-accidental trauma in pediatric patients: a review of epidemiology, pathophysiology, diagnosis and treatment. Transl Pediatr, 3, 195-207.
Maguire, S., Mann, M.K., Sibert, J. & Kemp, A. (2005). Are there patterns of bruising in childhood which are diagnostic or suggestive of abuse? A systematic review. Arch Dis Child, 90, 182-186.
Boos, S.C. (2017). Physical child abuse: Recognition. Retrieved April 21, 2017, from http://www.uptodate.com
Hobbs, C.J. (1986). When are burns not accidental? Archives of Disease in Childhood, 61, 357-361.
Binenbaum G., Rogers, D.L., Forbes, B.J., Levin, A.V., Clark, S.A., Christian C.W., Liu, G.T., & Avery R. (2013). Patterns of retinal hemorrhage associated with increased intracranial pressure in children. Pediatrics, 132, 430-434.
Litz, C.N., Ciesla, D.J., Danielson, P.D. & Chandler, N.M. (2017). A closer look at non-accidental trauma: Caregiver assault compared to non-caregiver assault. Journal of Pediatric Surgery, 52, 625-627.
Clearing C-Spine in Intoxicated Blunt Trauma Patients
Written by: Jason Chodakowski, MD (NUEM PGY-4) Edited by: Duncan Wilson, MD (NUEM ‘18) Expert commentary by: Matt Levine, MD
Saturday night in the ED. A 28 year old man presents after a low speed motor vehicle accident. Police report that he was seen swerving in the road before rear ending a parked car at approximately 25 mph. He presents to the ED without visible signs of trauma. His trauma exam reveals no cervical spine tenderness, but he is heavily intoxicated with a GCS of 13. Head CT and cervical spine CT are negative and he is currently sleeping in the hallway, periodically waking up to remove his cervical collar. You have very low suspicion that he has a significant cervical spine injury, but you ask yourself, can I clear his cervical spine given his level of intoxication?
Evaluating C-Spine Injuries
The Eastern Association for the Surgery of Trauma (EAST) Practice Management Guidelines Committee recommends the following approach to the care of patients with suspected cervical spine injuries: [1]
In awake, alert patients with trauma without neurologic deficit or distracting injury who have no neck pain or tenderness with full range of motion of the cervical spine, imaging is not necessary and the cervical collar may be removed.
All other patients in whom cervical spine injury is suspected should have radiographic evaluation, preferably with cervical spine CT imaging.
In patients with negative CT imaging but persistent neck pain, the patient may have a cervical ligamentous injury. Three treatment options exist:
Continue the cervical collar
Cervical collar may be removed after negative MRI
Cervical collar may be removed after negative and adequate flexion/extension plain films.
The Canadian C-Spine and National Emergency X-Radiography Utilization Study (NEXUS) criteria are two widely used, prospectively validated decision rules that can be used by clinicians to clinically rule out clinically significant cervical spine injury, thereby obviating the need for imaging.
Canadian C-Spine criteria [10]: If the patient has all of the below, then radiography is not necessary:
No High Risk Factors: Age >/=65; Dangerous Mechanism, paresthesias in extremities
AND has presence of at least one low risk factor: simple rear-end MVC, sitting position in ED, ambulatory at any time, delayed onset of neck pain, and absence of midline c spine tenderness
AND able to range neck actively (i.e. rotate neck 45 degrees left and right)
National Emergency X-Radiography Utilization Study (NEXUS) criteria [9]: If the patient meets all of the below criteria, no radiology is required.
No posterior midline cervical-spine tenderness
No evidence of intoxication
A normal level of alertness
No focal neurologic deficit
No painful distracting injuries
C-Spine Clearance in Intoxicated Patients
Intoxicated patients are an important population to consider in the setting of suspected cervical spine injury: not only do they make up nearly half of all blunt and penetrating trauma patients [2], but intoxication and reduced level of consciousness disqualify the use of the above decision-rules, thereby necessitating CT imaging. CT is insensitive for ligamentous injuries and current practice dictates that after a negative CT c-spine these patients (and obtunded patients generally) are left in a c-collar until they can be reassessed unaltered or have additional imaging performed, usually MRI.
A wealth of gradually accumulating data challenges the need to keep obtunded patients (and therefore plausibly intoxicated patients) in prolonged immobilization or to obtain MRI after a single negative CT c-spine, notably:
Smith et al [3]: meta-analysis, 16785 obtunded trauma patients
99.9% Sn and 99.9% Sp for CSI; NPV 100%
Panczykowski et al [4]: meta-analysis, 14327 obtunded or intubated patients
99.9% Sn and 99.9% Sp for unstable cervical spine injury
Patel et a [5]: systematic review, 1718 obtunded blunt trauma patients
NPV 100% for unstable CSI, 91% for any stable CSI
Raza et al [6]: meta-analysis, 1850 obtunded blunt trauma patients
93.7% Sn and 99%.7% Sp; NPV 99.7%
Hogan et al [7]: retrospective review, 1400 blunt trauma patients
NPV 98.9% for ligamentous injury; 100% for unstable CSI
EAST Practice Management Guidelines reflect these findings, conditionally recommending c-collar removal after a negative high-quality CT c-spine alone. [5]
Most recently, a prospective observational study of intoxicated patients with blunt trauma was published by Bush et al [8] in JAMA Surgery in 2016. The authors followed 1696 adult blunt trauma patients who underwent 2mm-thickness, three-view CT c-spine, finding that among intoxicated patients (alcohol or other drugs) a single negative CT c-spine alone had a NPV of 99.2% for all cervical spine injuries and 99.8% for unstable cervical spine injuries. Of the 632 intoxicated patients, only 1 had an unstable ligamentous injury that was missed on CT and later identified on MRI. This patient had quadriplegia on initial evaluation. The incidence and types of CSI were similar between intoxicated and sober groups.
Where Do We Go From Here?
Given the high incidence of intoxication in blunt trauma patients who are collared and require c-spine clearance, it is worth considering whether an otherwise neurologically intact intoxicated patient with a negative high-quality CT c-spine requires prolonged immobilization. This is of particular importance in patients that become combative and demand removal of their cervical collar. In such cases, ED physicians may be forced to sedate the patient in order to keep the cervical collar on or obtain an MRI, which may place the patient at risk. While the data is admittedly limited, it does demonstrate that the incidence of clinically significant c-spine injury in the setting of a negative CT scan is very low, with some authors stating it approaches zero. Given this, it may be justifiable to remove an intoxicated patient’s cervical collar in the setting of a reassuring clinical exam and negative CT scan in settings when the risk of keeping the patient in a cervical collar until sober is deemed to outweigh the risks of missed cervical spine injury.
Expert Commentary
Everything we recommend in medicine is a risk-benefit analysis. If there is extremely little to benefit, then do not recommend. If the risk exceeds the benefit, then do not recommend. Keep this in mind when considering various cervical collar scenarios, and the concept of being risk-averse vs being risk-neurotic.
Most of us think we are risk averse, but no one thinks they are risk neurotic. However, many witnessed practices regarding the use of cervical collars are exactly that. For instance, a patient that had an MVC yesterday presents with neck pain after waking up this morning. They have been moving all over, showered, dressed, etc. There is some midline tenderness so now they must lie flat and still and wear a collar. They are not allowed to walk, use the toilet, or move themselves onto a CT table even though they got themselves in and out of the car this morning. This is risk neurosis. This patient has gained nothing from wearing this collar. Furthermore, we have inconvenienced ourselves by having to now logroll this patient for imaging studies, not to mention the bedpan for the negative pregnancy test. Why are we doing this to ourselves and our patients? Risk neurosis.
Do not confuse this with the MVC patient who had immediate neck pain, was removed from the car by EMS and immediately placed in a collar. That patient has not moved around yet and declared themselves low enough risk yet. It is reasonable to handle them with care until the doctor can assess, and possibly image before considering collar removal. Risk averse.
Back to the intoxicated patient demanding collar removal. My risk-benefit calculator which is continuously churning in my head considers the two options:
Sedate and restrain a neurologically intact patient without signs of spine injury, despite not meeting strict clearance criteria due to intoxication. This puts him at risk for violent behavior, over sedation, aspiration, prolonged ED length of stay, etc. Even if the patient is hiding a fracture, is the struggle to restrain him protecting his spine or putting it at risk?
Do not make him wear the collar but make him stay in the ED until he can be clinically reassessed (when sober). Even if he has a c spine fracture, how likely is deterioration during this time?
Which is riskier for the patient’s spine, the restraint to force a collar on or the relatively peaceful collarless period? My risk-benefit calculator tells me the peaceful collarless period is safest.
So remember to ask yourself when faced with a cervical collar scenario what are the risks and benefits of applying this collar? Is there any real benefit? And then ask yourself the truly difficult but introspective question: Am I being risk averse or am I being risk-neurotic?
Matt Levine, MD
Assistant Professor of Emergency Medicine
Northwestern Feinberg School of Medicine
Citations
Como, John J., et al. "Practice management guidelines for identification of cervical spine injuries following trauma: update from the eastern association for the surgery of trauma practice management guidelines committee." Journal of Trauma and Acute Care Surgery 67.3 (2009): 651-659.
Rivara, Frederick P., et al. "The magnitude of acute and chronic alcohol abuse in trauma patients." Archives of Surgery 128.8 (1993): 907-913.
Smith, Jackie S. "A synthesis of research examining timely removal of cervical collars in the obtunded trauma patient with negative computed tomography: an evidence-based review." Journal of Trauma Nursing 21.2 (2014): 63-67.
Panczykowski, David M., Nestor D. Tomycz, and David O. Okonkwo. "Comparative effectiveness of using computed tomography alone to exclude cervical spine injuries in obtunded or intubated patients: meta-analysis of 14,327 patients with blunt trauma: A review." Journal of neurosurgery 115.3 (2011): 541-549.
Patel, Mayur B., et al. "Cervical spine collar clearance in the obtunded adult blunt trauma patient: a systematic review and practice management guideline from the Eastern Association for the Surgery of Trauma." The journal of trauma and acute care surgery 78.2 (2015): 430.
Raza, Mushahid, et al. "Safe cervical spine clearance in adult obtunded blunt trauma patients on the basis of a normal multidetector CT scan—a meta-analysis and cohort study." Injury 44.11 (2013): 1589-1595.
Hogan, Gerard J., et al. "Exclusion of Unstable Cervical Spine Injury in Obtunded Patients with Blunt Trauma: Is MR Imaging Needed when Multi–Detector Row CT Findings Are Normal? 1." Radiology 237.1 (2005): 106-113.
Bush, Lisa, et al. "Evaluation of cervical spine clearance by computed tomographic scan alone in intoxicated patients with blunt trauma." JAMA surgery 151.9 (2016): 807-813.
Hoffman, J.R., et. al. “Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group.” NEJM. 2000. 343(2):94-99.
Stiell, IG, et. al. “The Canadian C-Spine rule for Radiography in Alert and Stable Patients.” JAMA. 2001. 286(15):1841-8.
How To Cite This Post
[Peer-Reviewed, Web Publication] Chodakowski J, Wilson D. (2019, Sept 16). Clearing C-Spine in Intoxicated Blunt Trauma Patients. [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/cspine-clearance-etoh.
Other Posts You May Enjoy
Pelvic Fractures
Written by: Justine Ko, MD (NUEM PGY-3) Edited by: Terese Whipple, MD (NUEM PGY-4) Expert commentary by: Matt Levine, MD
Pelvic Ring Fractures
Overview
Pelvic ring fractures make up about 3% of skeletal fractures [1]. In the emergency department, they are often seen with high-impact blunt trauma, such as MVCs, crush injuries, or falls.
The pelvis consists of the sacrum and the two innominate bones, which are made up of the ilium, ischium, and pubis [2]. The sacrum and the innominate bones together form a posterior arch and an anterior arch that join to make a ring, also known as the pelvic inlet. The stability of the pelvis is enforced by ligaments: the sacroiliac ligaments, the sacrospinous ligaments, and sacrotuberous ligaments [2]. The ligaments and bones together give the pelvis vertical and rotational stability.
Pelvic ring fractures carry a significant mortality, ranging from 15-25% in closed fractures, and as much as 50% in open fractures [3]. They are often associated with other high-mortality injuries as well, such as chest injuries, spinal injuries.
What are the mechanisms associated with these injuries?
The Young-Burgess classification system categorizes injury patterns based on the mechanism of injury and the forces applied: anteroposterior compression (APC), lateral compression (LC), and vertical shear (VS) [1]. The most common mechanism is lateral compression, which occurs when lateral forces are applied to the pelvis (as the name would suggest). APC injuries result in the classic “open book” pelvic fractures (Figure 1).
Figure 1
Figure 2
Anteroposterior compression: causes disruption of the pubic symphysis. A normal pubic symphysis should not exceed 5mm. Injuries where the symphysis widening is <2.5cm, with no significant posterior column injury or other fractures, are usually considered stable. APC injuries are often associated with significant blood loss, as the pelvic volume increases and can store more blood [4].
Lateral Compression: considered rotationally unstable. This mechanism causes the pelvic volume to decrease, and therefore, is associated with less blood loss [4].
Vertical shear: the most unstable, often associated with axial loading of the hemipelvis, such as with a fall or a jump from a height.
What exam findings should I look for?
During a trauma workup, visual inspection for leg-length discrepancy or rotation of the pelvis may provide clues to a hip or pelvic injury. You may assess for stability of the pelvis by applying downward and medial pressure to the bilateral iliac crests. Apply pressure gently and slowly to avoid disruption of any possible clots that may have formed.
A digital rectal exam should also be performed to assess for spinal cord injury and frank blood. In males, the meatus should be assessed for possible bladder or urethral injury.
X-ray reading tips
Check the 3 circles! (Figure 3)
Pubic inlet
Two obturator foramina
Check for irregularities along the circles
In general, a vertical fracture pattern will be associated with APC or VS mechanisms. Horizontal fracture patterns will be associated with LC. [7]
Figure 3
Check the lines!
ilioischial line (orange line in Figure 4)- disruption suggest posterior column fracture [5]. The posterior column involves the posterior half of the acetabulum and extends to the ischial tuberosity [4].
iliopectineal line (red line in Figure 4)- disruption suggests anterior column fracture [5]. The anterior column involves the anterior half of the acetabulum and extends to the lateral half of the superior pubic ramus [4].
It’s a circle! If it breaks in one location, it likely broke in another place. Be wary of isolated rami fractures in the setting of high-impact trauma!
Figure 4
Management
As always, still stick to your ABCs!
Initiate blood transfusion early for patients in shock
Hemodynamic instability occurs in about 10% of these injuries [1]
Try to obtain IV access above the pelvis if a pelvic fracture is suspected.
Stabilize the pelvis with a TPOD or a sheet for patients with open book pelvic fractures
Apply over the GREATER TROCHANTERS
Do not apply a compressive device for isolated lateral compression pelvic fractures as the device can cause further compression and worsen the displacement [4].
If the hemorrhage or hypotension is suspected to be from pelvic hemorrhage, activate IR early.
If stable, obtain a CTA of the pelvis to assess for vascular injury
What other injuries should I look for?
Urologic Injury: incidence in pelvic fractures is ~5%. Patient with blood at the meatus will need a retrograde urethrogram and then a cystogram [4].
Neurologic Injury: pelvic fractures can be associated with plexus injuries and even cauda equina syndrome. Check for rectal tone and saddle anesthesia [4].
Gynecologic injury: blood a the introitus can represent urethral injury or an open pelvic fracture [4].
Expert Commentary
Dr Ko has provided a high yield concise guide of the key cognitive and clinical features of pelvic fractures. A few points worthy of further emphasis:
When examining for pelvic instability, do not rock on the pelvis to further open it. Instead, compress medially to feel instability while closing, rather than opening, the pelvis.
For most studies we order, we have the luxury of waiting for a formal report. Pelvis x rays are one of the few radiographic studies that the Emergency Physician must be able to rapidly interpret independently at the bedside for patient management! Do not just eyeball the film. It is impossible to find multiple pelvic fractures that way. Go over every arc, curve, and ring visually. If something appears possibly abnormal, compare to the other side for (a)symmetry. And if you find one break in the pelvic ring, scrutinize for more, there almost always are. Many of these are in the posterior ring, the part of the pelvis for which plain films are least sensitive. CT will help evaluate the posterior elements if feasible.
Understanding the classic LC, APC, VS mechanism classifications lend insight into when a binder will help. Realize, however, that in vehicular trauma, often the forces are multidirectional, so there will be mixed injury patterns. Ask yourself what you are trying to achieve by placing the binder. If it is closing the open book to tamponade hemorrhage, then go for it! If it is to provide some stability to a VS injury so you can move the patient without their pelvis flopping around than OK but it won't be therapeutic. If it is an LC injury where the femoral head has crushed through the acetabulum, avoid using a binder, you will only further intrude bony fragments into the pelvis!
Call IR the instant you realize there is a bleeding pelvic fracture. It takes time to prepare the suite, especially overnight!
Matt Levine, MD
Assistant Professor of Emergency Medicine
Northwestern Feinberg School of Medicine
How To Cite This Post
[Peer-Reviewed, Web Publication] Ko J, Whipple T. (2019, July 22). Pelvic Fractures. [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/pelvic-fracture.
Other Posts You May Enjoy
References
Halawi MJ. Pelvic ring injuries: Emergency assessment and management. Journal of Clinical Orthopaedics and Trauma. 2015;6(4):252-258.
Khurana B, Sheehan SE, Sodickson AD, Weaver MJ. Pelvic ring fractures: What the orthopedic surgeon wants to know. RadioGraphics. 2014;34:1317-1333
Weatherford B. Pelvic ring fractures. Orthobullets website. https://www.orthobullets.com/trauma/1030/pelvic-ring-fractures. Accessed October 14, 2018.
Walls RM, Hockberger RS, Gauche-Hill M. (2018). Rosen's emergency medicine: Concepts and clinical practice (9th ed.). Philadelphia, PA: Elsevier/Saunders.
Murphy A, Jones J. Pelvic radiograph (an approach). Radiopaedia website. https://radiopaedia.org/articles/pelvic-radiograph-an-approach. Updated August 2018. Accessed October 14, 2018.
Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br Vol. 1988;70(1):1–12.
Singh, AP. Pelvic fractures: Presentation and treatment. https://boneandspine.com/pelvic-fractures-presentation-and-treatment/
Images:
Case courtesy of Dr Jeremy Jones, https://radiopaedia.org Radiopaedia.org. From the case https://radiopaedia.org/cases/28928" rID: 28928
C-spine Clearance with Negative CT: Are We There Yet?
Written by: M. Terese Whipple, MD (NUEM PGY-3) Edited by: Quentin Reuter, MD (NUEM ‘18) Expert commentary by: Matthew Levine, MD
We have excellent decision rules for clinically clearing cervical spine injury in low risk patients without imaging. However, a frustrating situation arises when a CT of their c-spine is obtained and negative, but they are having persistent midline pain. What do we do then? Are we forced to order an MR of the c-spine even when they have no neurological deficits and our gestalt tells us there is no clinically significant injury? MR often means admission, worsening of already overwhelming ED crowding, and unhappy patients when they cannot remove the c-collar for at least several more hours. Recent data and recommendations suggest that this may not be the case; a negative CT may be enough to rule out clinically significant injury. This blog post will explore some of the historical and recent data on the subject of cervical spine clearance after CT scan alone.
There has been great historical debate over the best management for patients with persistent midline pain after negative CT, however that evidence is beyond the scope of this post. Current common practice and the recommendation of the American College of Radiology leads down the path of cervical spine MR when this situation arises [1]. Due to the cumbersome logistics of MR, much work has been done to determine if MR truly adds value to the patient’s workup. Is MR catching clinically significant injury missed by CT that changes clinical management? The majority of studies have concluded that the answer to that question is no.
In 2015 the Eastern Association for the Surgery of Trauma (EAST) sought to tackle this question by reviewing all studies to date examining C-spine evaluation in obtunded patients [2]. They evaluated 11 studies with a total of 1718 obtunded patients who underwent C-Spine imaging with CT. None were ultimately found to have unstable fractures or unstable ligamentous injury missed by CT. There was a 9% incidence of stable injuries missed on CT and found on follow up MR, flex-x, upright XR, or clinical follow up. They found a cumulative 100% NPV for unstable C-Spine injury with CT and 91% NPV for stable injury. They did rate the quality of evidence as low for various reasons, including non-comparable imaging protocols, inconsistently reported and variable outcomes, publication bias, and an overall inability to perform a meta-analysis with the data. However, they rated the data from which they derived the NPV as moderate quality as the NPV was consistently 100% throughout all of the trials. Based on their analysis they provided the following recommendation for obtunded blunt trauma patients:
“We conditionally recommend cervical collar removal after negative high-quality c-spine CT scan results alone.”
They went on to further clarify,
“It should be acknowledged that cervical collar removal can result in neurological change and even paralysis, although this may be underreported in the literature. However we cannot continue indiscriminate two-stage sequential screening for C-spine injuries if the injury rate is near 0% for the first test and the second adjunctive test results in false positives and inconsistent treatment plans.”
But the real question that is more pertinent to us as EM physicians (obtunded MR’s are usually dealt with upstairs), is: if we can remove the c-collars of obtunded patients after negative CT, why couldn’t that be extrapolated to awake patients? Well, they commented on that too:
“Therefore, if collars are to be removed in a high risk obtunded population […] cervical collar removal can be logically argued for any population-obtunded or not.” [2]
They finally call for multicenter prospective research on the subject, again citing the low quality of evidence that they used for their recommendation. That call was answered in 2017 by the Western Trauma Association. The group completed a multi institution trial with 10,000 patients who were getting a CT for evaluation of cervical spine injury prospectively enrolled at 17 centers [3]. They found only 3 CT scans that missed clinically significant injury (.03%). All of those patients had focal neurological abnormalities on exam. There was no clinically significant injury missed by CT and exam combined. CT scan alone had an NPV of 99.97%, and an NPV of 100% when combined with clinical exam. Therefore, they proposed this diagnostic algorithm:
Most trials have found similar results, with a few exceptions. Two trials prior to the publication of the Western Trauma Association (WTA) paper found that CT missed a few clinically significant injuries in patients with no neurological symptoms. Both trials enrolled significantly fewer patients than the WTA paper, and only enrolled patients with negative CT who would be evaluated with MR, meaning they couldn’t comment on the overall sensitivity of CT in unstable c-spine injury. The ReConect trial in 2016 found 5 of 767 patients (.6%) with injuries requiring surgical intervention that were missed on CT [4]. Another study with similar methods published in Annals of Emergency Medicine in 2011 evaluated those who had a negative CT but were MR’ed for persistent midline C-Spine tenderness [5]. They found that out of 178 patients, 5 had injury requiring operative management that was missed on CT but found on subsequent MR (2.8%) [5]. The Annals paper is certainly an outlier, with a considerably higher rate of missed clinically significant injury than the remainder of the literature, with rates usually between 0-1% [6-18]. The authors believe this may be due to more stringent methodology. For instance, they required MR to be performed within 48 hours when it is the most sensitive for edema, and only enrolled patients with midline tenderness rather than subjective pain [5]. While this may be true, the results have not been replicated in subsequent studies.
With the publication of the WTA paper, evidence certainly seems to be tipping in favor of CT clearance of cervical spine in neurologically intact patients. However, a few questions remain. In every study discussed here, MR resulted in discharge with hard collar in a portion of patients. Indications ranged from stable injury to persistent pain with no evidence of injury on MR. It is unclear whether hard collar placement makes a difference in the clinical course of these patients, if their stable injuries would have become unstable without it, or if it has any long term impact on outcomes such as chronic pain. This is an important question not yet adequately addressed in the literature. The majority of these trials were also completed at trauma centers with radiologists well trained in reading c-spine imaging and high quality CT scanners. It could be difficult to generalize this data to centers with older scanners or whose radiology departments are not as expert in trauma radiology.
Incredibly high quality and reproducible evidence is required to change practice when high stakes, such as potentially missed cervical spine injury, are involved. So far we have multiple trials showing an NPV of close to 100% when CT and good neurological exam are combined, and the conditional recommendation by the EAST group. Time will tell if recommendations in the future remove the “conditional” portion as CT technology continues to improve, further studies with stringent methodologies are conducted, and the results of the WTA paper are hopefully replicated.
Expert Commentary
Thank you Dr Whipple for that really practical review of a real-life common clinical question we face all the time: Can we remove the collar? Some important takeaways are:
There is a robust and growing body of evidence that removing the collar after a negative high-quality CT is safe if the patient is neurologically intact.
This practice is endorsed by two major trauma organizations, EAST and WTA.
The endorsement by respected major trauma societies is important in translating evidence into practice. It seems like all that is left at this point for widespread implementation is overcoming culture. This would likely require addressing the outlier studies listed by Dr Whipple to win over those still skeptical. Part of overcoming culture would involve buy-in from neurosurgical societies. What do neurosurgical societies say regarding clearing these patients? There are many instances in which a patient is discharged with recommendations from the neurosurgeon to wear a hard collar despite a negative CT and MRI. On the surface this seems like defensive medicine and impractical for the patient. Is the patient really going to comply with this until follow up? Is this collar really protecting them and preventing further injury which, after negative CT and MRI and with a normal neuro exam, seems exceedingly unlikely? Does evidence support this practice?
In the end, decision rules should be used when you want evidence to support your clinical decisions, such as removing the C collar after negative imaging in a neurologically intact patient. Do not use decision rules, however, to overturn or replace sound clinical judgement. If there is something about a case that makes you still feel like you could be missing an outlier injury by removing the collar, listen to that voice inside of you. It is that sound clinical judgement that will guide you through your career, not decision rules.
Matthew Levine, MD
Northwestern Medicine, Assistant Professor of Emergency Medicine
How To Cite This Post
[Peer-Reviewed, Web Publication] Whipple T, Reuter Q. (2019, May 13). C-spine clearance with negative CT: Are we there yet? [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/cspine-clearance-ct
Other Posts You May Enjoy
References:
American College of Radiology. ACR appropriateness criteria on suspected spine trauma. Available at: http://www.acr.org.
Patel MB, et al. Cervical spine collar clearance in the obtunded adult blunt trauma patient: A systematic review and practice management guideline from the Eastern Association for the Surgery of Trauma. J Acute Care Trauma Surgery. 789(2): 432-441.
Inaba, K et al. Cervical Spine Clearance: A Prospective Western Trauma Association Multi-Institutional Trial. J Trauma Acute Care Surg. 2016 Dec: 81(6): 1122-1130.doi: 10.1097/TA.0000000000001194
Maung A, et al. Cervical spine MRI in patients with negative CT: A prospective, multicenter study of the Research Consortium of New England Centers for Trauma (ReCONECT). J Trauma Acute Care Surg. 82 (2): 263-269.
Ackland HM, et al. Cervical Spine Magnetic Resonance Imaging in Alert, Neurologically Intact Trauma Patients With Persistent Midline Tenderness and Negative Computed Tomography Results. Ann of Em Med. 2011 Dec. 58 (6): 521-530.
Chew B, et al. Cervical spine clearance in the traumatically injured patient: is multidector CT scanning sufficient alone? J Neurosurg Spine. 2013. 19: 576-581
Bush L, et al. Evaluation of cervical spine clearance by computed tomographic scan alone in intoxicated patients with blunt trauma. JAMA Surg. 2016; 151 (9): 807-813
D’Alise et al. Magnetic resonance imaging for the evaluation of the cervical spine in the comatose or obtunded trauma patient. J Neurosurgery (Spine 1) 1999; 91:54-59.
Resnick S, et al. Clinical relevance of magnetic resonance imaging in cervical spine clearance: a prospective study. JAMA Surg. 2014; 149 (9): 934-9.
Menaker J, Philp A, Boswell S, Scalea TM. Computed tomography alone for cervical spine clearance in the unreliable patient--are we there yet? J Trauma. 2008; 64(4):898–903.
Chew BG, Swartz C, Quigley MR, Altman DT, Daffner RH, Wilberger JE. Cervical spine clearance in the traumatically injured patient: is multidetector CT scanning sufficient alone? Clinical article. J Neurosurg Spine. 2013; 19(5):576–81.
Como JJ, Leukhardt WH, Anderson JS, Wilczewski PA, Samia H, Claridge JA. Computed tomography alone may clear the cervical spine in obtunded blunt trauma patients: a prospective evaluation of a revised protocol. J Trauma. 2011; 70(2):345–9. discussion 9-51.
Khanna P, Chau C, Dublin A, Kim K, Wisner D. The value of cervical magnetic resonance imaging in the evaluation of the obtunded or comatose patient with cervical trauma, no other abnormal neurological findings, and a normal cervical computed tomography. J Trauma Acute Care Surg. 2012; 72(3):699–702.
Schuster R, Waxman K, Sanchez B, Becerra S, Chung R, Conner S, Jones T. Magnetic resonance imaging is not needed to clear cervical spines in blunt trauma patients with normal computed tomographic results and no motor deficits. Arch Surg. 2005; 140(8):762–6.
Anekstein Y, Jeroukhimov I, Bar-Ziv Y, Shalmon E, Cohen N, Mirovsky Y, Masharawi Y. The use of dynamic CT surview for cervical spine clearance in comatose trauma patients: a pilot prospective study. Injury. 2008; 39(3):339–46.
Brohi K, Healy M, Fotheringham T, Chan O, Aylwin C, Whitley S, Walsh M. Helical computed tomographic scanning for the evaluation of the cervical spine in the unconscious, intubated trauma patient. J Trauma. 2005; 58(5):897–901.
Harris TJ, Blackmore CC, Mirza SK, Jurkovich GJ. Clearing the cervical spine in obtunded patients. Spine (Phila Pa 1976). 2008; 33(14):1547–1553.
Steigelman M, Lopez P, Dent D, Myers J, Corneille M, Stewart R, Cohn S. Screening cervical spine MRI after normal cervical spine CT scans in patients in whom cervical spine injury cannot be excluded by physical examination. Am J Surg. 2008; 196(6):857–862.
Unstable Cervical Spine Fractures
Written by: Sarah Sanders, MD (NUEM PGY-4) Edited by: Alison Marshall, MD (NUEM Alum ‘17) Expert commentary by: Steve Hodges, MD
Fractures of the cervical spine are injuries that must be approached with caution. Some are stable, some are unstable, and mismanagement can lead to life-altering sequelae. Remembering which fractures fit into which category is imperative for optimum emergency department care.
A quick review of cervical spine anatomy is a helpful starting point:
All the cervical spine anatomy images are credited to Netter, FH Atlas of Human Anatomy, Sixth Edition.
All the cervical spine anatomy diagrams are credited to Agur, AMR & Dalley, AF of Grant’s Atlas of Anatomy: Twelfth Edition.
The common mnemonic “Jefferson Bit Off A Hangman’s Thumb,” is used to remember the unstable fractures, which will be reviewed in this post.
Ultimately, understanding the mechanism of injury is crucial in identifying and accurately managing these injuries. The below PV cards are organized by mechanism and tailored down to be an on-shift reference.
In conclusion, cervical spine injuries required a high index of suspicion and caution by the emergency medicine physician as their variability and potential for neurological impairment is high. Hopefully this review can provide you with additional insight and ease of memory when the next Level 1 trauma rolls through the door.
Expert Commentary
Thanks for this insightful post. You've done a really nice job in laying out the most salient points. It is important to have an understanding of the anatomy and this is a great review. Knowing the factors that put people a high risk for injury is also paramount. Certain chronic disease states and anatomic variances, as you note, do put select patient populations at risk for specific injury. The mechanism of injury is something we always talk about, but when it comes to neck trauma we need to really pay attention to all available history including paramedic reports, or even cell phone video, to get the best possible picture or the mechanism of injury. I can not stress enough the importance of a detailed neurological exam including sensation(s) and reflexes. Any asymmetric finding should raise your level of suspicion for severe injury. Moving to advanced imaging is especially important if there is a complaint of a focal neurological deficit; be that transient, subjective or blatantly obvious.
Athletes or motorcyclist who have suspected cervical spine injury, who have protective shoulder pads and/or helmets pose a unique challenge. Eventually the protective devices are going to need to be removed. There are many opinions on how best to do this and whether an x-ray should be done before attempting the removal of protective gear. From personal experience; it can be difficult to remove protective gear; I recommend getting "all hands on deck" and using an methodical slow organized approach. More recent thought is that cervical spine imaging should incorporate procedures for removal of equipment before initial radiographic evaluation.[1] Once the gear is removed a c-collar should then be applied and you can proceed with imaging.
Recommendations for imaging the cervical spine for trauma has changed quit a lot over the last several years. The National Emergency X-Radiography Utilization Study (NEXUS) and the Canadian C-Spine Rule (CCR) have been validated and have allowed our practice to advance such that we can effectively practice clinical medicine. However, a word of caution on using these criteria with patients who could be impaired. Sometimes the mild dementia, delirium or subtle drug, alcohol intoxication can lead us astray when we rely solely on these criteria. The cross table lateral films and specifically flexion/extension views have fallen out of favor. Most patients without focal neurological complaint or deficit are imaged with plain CT. If your patient has a focal neurological complaint or deficit, a suspected ligamentous or disc injury an MRI should be done. Depending on the exam and risk factors I would consider either a CTA or and MRA to evaluate for vascular injury.
You asked about c-collars specifically. What is available to you will be somewhat hospital - vendor specific. I prefer the Aspen or the Miami collar, they are very similar in function overall and superior to the pre-hospital EMS ones. When a patient has to be transported to another facility; make sure that the patient has full immobilization with back board and head-side blocks with the collar and head secured to the side blocks. An immobilized patient that requires intubation can make an easy air way difficult and a difficult airway terrifying. Make sure you have all your equipment prepared, including a surgical method, before you intubate. The person holding c spine immobilization needs to knows their role.....don't let go and don't move. This is the time when a video assisted intubation should be used. Use either the intubating bronchoscope or a video laryngoscope. This article talks a bit about managing airway in cervical spine injury and is a nice reference.[2]
Closing thoughts: maintain a high level of suspicion for injury in the setting of a focal neurological deficit, immobilize early immobilize often and don't be shy about intubating before transferring.
https://doi.org/10.1067/mem.2001.116333 Annals of Emergency Medicine; Baldwin et. al., "Football protective gear and cervical spine imaging" July 2001 Volume 38, Issue 1, Pages 26–30
10.4103/2229-5151.128013 International Journal of Critical Illness and Injury Science; Austin et. al., "Airway management in cervical spine injury" Jan-Mar; 4(1): 50–56
Steven W. Hodges, MD, FACEP
Assistant Medical Director
Northwestern Lake Forest Hospital
How To Cite This Post
[Peer-Reviewed, Web Publication] Sanders S, Marshall A (2019, January 21). Unstable Cervical Spine Fractures [NUEM Blog. Expert Commentary by Hodges S]. Retrieved from http://www.nuemblog.com/blog/cervical-spine-fractures.
Other Posts You May Enjoy
References
Adams, J. Lin, M. Mahadevan, SV. “Spine Trauma and Spinal Cord Injury.” Section VIII, Chapter 75. Emergency Medicine: Clinical Essentials. Second Edition. P652 - 657.
Agur, AMR; Dalley AF. Grant’s Atlas of Anatomy. Twelfth Edition. Chapter 4: Back. 2009.
Bergenheim, AT, Forssell, A. “Vertical Odontoid Fracture. Case Report.” Journal of Neurosurgery. Vol 74 (4) p665-667. 1991.
Netter, F. Atlas of Human Anatomy. Section Head & Neck. Sixth Edition. 2014.
Wheeless, CR. “Cervical Spine.” Wheeless Textbook of Orthopedics by Duke University. April 26 2016. 2 Jan 2017. http://www.wheelessonline.com/ortho/cervical_spine
REBOA
Written by: Andew Cunningham, MD (NUEM PGY-4) Edited by: Bill Burns, MD (NUEM Alum ‘17) Expert commentary by: Zaffer Qasim, MBBS, FRCEM, FRCPC(EM), EDIC
REBOA: Ready for Prime Time?
For years, the resuscitative thoracotomy has been the sole weapon in the physician’s arsenal against a loss of a perfusing pressure in the crashing trauma patient. With the advent of new endovascular technologies, novel methods to control hemorrhage are being refined, among them Resuscitative Endovascular Balloon Occlusion of the Aorta or REBOA. With this newer method getting a lot of attention in the emergency and trauma communities, it’s time to take a look at what it is, how successful it is, and where we are going with it.
What Is It?
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a possible alternative to resuscitative thoracotomy in cases of non-compressible torso hemorrhage (NCTH) that present to the Emergency Department in extremis.1
REBOA works via the insertion of a catheter through the femoral artery to allow an endovascular balloon to be deployed within the aorta, allowing for bleeding control and augmentation of afterload in hemorrhagic shock.2
REBOA can be deployed in Zone III of the Aorta, as depicted in the image below, for pelvic hemorrhage, or in Zone I of the Aorta for abdominal hemorrhage.3
Borrowed from Reference 3
The primary indications for REBOA include:
PEA arrest secondary to abdominal or pelvic hemorrhage within 10 minutes of the onset of arrest
Severe hypovolemic shock secondary to abdominal or pelvic hemorrhage
Unstable hemodynamics refractory to volume resuscitation in patients with abdominal or pelvic hemorrhage2
The major contraindications include age older than 70, significant comorbidities, prolonged PEA arrest (lasting longer than 10 minutes), or high suspicion for proximal aortic injury (REBOA may exacerbate bleeding from thoracic sources).2
Does It Work?
A study at U of Arizona showed that 45% of patients who received thoracotomy may have benefited from REBOA based on autopsy results, but only 32% of the patients receiving a thoracotomy did not have a contraindication for it, and of those who did not have a contraindication, only roughly half would have potentially benefited. Compared to prior literature, this may suggest that REBOA is not as useful in patients in extremis.1
Although the literature does suggest that REBOA reduces the amount of overall hemorrhage, there is still no definitive evidence in humans of a decrease in mortality.4
There are still risks of complications in humans, including arterial injury and limb ischemia.3 In animal models, REBOA has also resulted in renal failure, liver failure, intestinal ischemia, and multiple other injuries which result from occlusion of the aorta.5
Given that REBOA still obstructs distal flow, just like cross-clamping the aorta in a resuscitative thoracotomy, it is still reserved as a last resort maneuver. The effects of aortic occlusion can be reviewed below6:
Borrowed from Reference 6
Where Is It Going?
An alternative to REBOA may be Selective Aortic Arch Perfusion (SAAP); in this similar yet separate endovascular approach, a catheter that has two ports is utilized instead of the single-port REBOA catheter. This allows for both occlusion of the aorta and selective administration of blood, pressors, or other medications directly to the heart and brain. Where REBOA may be useful for exclusively shock, SAAP may have advantages in cardiac arrest secondary to trauma.7
A new, smaller REBOA catheter, the 7 French ER-REBOA, may cause fewer injuries and also allows for simultaneous blood pressure monitoring.8
Partial REBOA, or P-REBOA, allows for controlled blood flow to the body distal to the area of occlusion, in efforts to limit ischemia.5
Is It Feasible for ER Docs to Perform?
Yes! Some of the larger studies performed in Japan required placement by either a surgical or emergency medicine-trained attending.9
In England, there are case of REBOA being deployed in the pre-hospital setting to act as a bridging method for resuscitation during transport.7 As the relay between the hospital and Emergency Medical Services, it is an EM physician’s responsibility to be aware of this method and its utility in her area.
Take-Home Points
REBOA is a newer up-and-coming method of controlling hemorrhage secondary to abdominopelvic trauma that may act as an alternative to resuscitative thoracotomy.
Although more data still needs to be collected, REBOA has not yet shown to clearly improve mortality, and does come with certain risks and complications.
There are more novel methods of REBOA undergoing research and development, including SAAP, ER-REBOA, and P-REBOA, which may strengthen the utility of REBOA and reduce some of the complication risks.
REBOA is within an EM physician’s scope of practice, and may play a role in EMS in the future. As such, it is our duty to be aware of it and follow along with its developments.
Expert Commentary
Thanks for a great post on an evolving temporary hemorrhage control concept. Hemorrhage, and torso hemorrhage in particular, remains the largest cause of death in trauma in the first 24 hours. In the right patient, REBOA can be another effective procedure in the emergency physician’s toolbox. Some additional points to consider:
1. Access is key! The rate limiting step is early common femoral artery (CFA) access. It’s important to emphasize accessing the common as placing the sheath in one of the smaller branch vessels could increase the risk of iatrogenic injury. I advocate using ultrasound to define the anatomy and routinely placing a CFA arterial line in your “big sick” patients to maintain skills. As you state, this step is well within the wheelhouse of the Emergency Physician, and the foundation to build on to train in placing REBOA
2. Patient selection is critical! The available data generally has the inclusion/exclusion criteria listed, but definitions on who is “unstable” vary. In my opinion, an arbitrary blood pressure cutoff of <90mmHg in someone with torso hemorrhage should not automatically trigger REBOA. I think these patients should get a CFA line, and then proceed to REBOA only if not responding to initial resuscitative measures or rapidly deteriorating to imminent arrest.
3. Placement before arrest will likely lead to better outcomes. The evolving data shows that the group that benefits most in terms of mortality are the nonresponders who will imminently arrest unless they have a lifesaving procedure. In the arrested patient, as mentioned, determining the time of arrest is crucial. This can certainly be challenging with prehospital arrest.
4. While the data does not show improved mortality compared to thoracotomy, there does seem to be a trend to improved neurologically intact survival – this is our ultimate goal and speaks to the ability to use REBOA proactively, before traumatic arrest happens
5. It is absolutely critical that REBOA is used in a system that can rapidly deliver these patients to definitive care (OR/IR). The consequences of prolonged balloon occlusion as listed are dire. Based on collective clinical experience and translational animal data, I would not recommend occluding beyond 45 minutes to 1 hour in Zone 1.
6. REBOA in the US is currently used only at level 1 and 2 trauma centers. I think (as the British have shown), the biggest benefit is likely at smaller centers and ultimately prehospital. Success here will be based on procedural considerations (like p-REBOA to prolong safe inflation times), appropriate training, and systems issues (expedited transfer to definitive care).
Zaffer A. Qasim, MBBS, FRCEM, FRCPC(EM), EDIC
Assistant Professor of Clinical Emergency Medicine
UPenn Medicine
How to Cite This Post
[Peer-Reviewed, Web Publication] Cunningham A, Burns W (2019, January 7). REBOA [NUEM Blog. Expert Commentary by Qasim Z]. Retrieved from http://www.nuemblog.com/blog/REBOA
Other Posts You May Enjoy
References
Joseph B, Ibraheem K, Haider AA, et al. Identifying potential utility of resuscitative endovascular balloon occlusion of the aorta: An autopsy study. J Trauma Acute Care Surg. 2016;81:S128-S132.
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Vol 2016. LIFTL.
Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin. 2017;33:55-70.
Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80:324-334.
Perkins ZB, Lendrum RA, Brohi K. Resuscitative endovascular balloon occlusion of the aorta: promise, practice, and progress? Curr Opin Crit Care. 2016;22:563-571.
Russo RM, Neff LP, Johnson MA, Williams TK. Emerging Endovascular Therapies for Non-Compressible Torso Hemorrhage. Shock. 2016;46:12-19.
Bebarta V. REBOA - Ready for Prime Time? ACEP EM Education.
Weingart S. Podcast 170 - the ER REBOA Catheter with Joe DuBose. Vol 2016. EMCrit Blog.:Available at [http://emcrit.org/podcasts/er-reboa].
Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78:897-903; discussion 904.
Topical Hemostatics
Written by: Alex Ireland, MD (NUEM PGY-3) Edited by: Andrew Moore, MD (NUEM Alum ‘18) Expert commentary by: Joseph Posluszny, MD
Expert Commentary
The above summary of the mechanism of actions, indications for and limitations of topical hemostatic agents is comprehensive and thorough.
As with most summaries of hemostatic agents, we focus on the mechanism of action or aspect of coagulation by which the agent works. Clinically, it may be easier to take a different approach.
Sometimes, the most difficult aspect of applying a topical hemostatic agent is determining the appropriate agent given the clinical scenario- all work in some fashion, but which will work best?
To help approach this, an initial question to help frame what to do in the trauma bay or ED would be: does this wound require just a hemostatic agent as a covering/dressing to promote hemostasis or are both assisted hemostasis and pressure needed to control the bleeding? For superficial, low volume, but persistently bleeding wounds, topical agents like Dermabond, thrombin (with gelfoam) and Surgicel are ideal. For deep, complex wounds that require hemostatic agents and pressure, QuikClot Gauze, Ativene and Combat Guaze are more effective.
Hospitals and EMS systems purchase a variety of topical hemostatic agents. It is imperative to become familiar with these agents and be prepared for their indications before you are presented with bleeding uncontrolled by conventional dressings or pressure.
Joseph Posluszny, MD
Assistant Professor of Surgery
Trauma and Critical Care, McGaw Medical Center of Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Ireland A, Moore A (2018, December 10). Topical Hemostatics [NUEM Blog. Expert Commentary by Posluszny J]. Retrieved from http://www.nuemblog.com/blog/topical-hemostatics
Bruised and broken hearts: diagnosis and management of blunt cardiac injury
Written by: Paul Trinquero, MD (NUEM PGY-4) Edited by: Victor Gappmaier, MD (NUEM Alum '18) Expert commentary by: Emily Koeck, MD
Clinical vignette
A 39-year-old male presents as a fall from a two-story window, landing on his left side. He lost consciousness after the fall but is now back to his baseline mental status. Primary survey is intact and his GCS is 15. Secondary survey is notable for a left temporal scalp hematoma and tenderness over his left anterior chest. A CT brain and CT cervical spine are obtained and both are unremarkable. CT chest is notable for two left sided rib fractures and a small underlying pulmonary contusion, without any evidence of hemothorax or pneumothorax. Given his high-risk mechanism for blunt cardiac injury, ECG and troponin are obtained. Troponin is negative but ECG demonstrates a right bundle branch block, with no prior for comparison. The patient remains well appearing and hemodynamically stable. He is asymptomatic other than mild chest wall pain. This typical multiple blunt trauma patient raises some interesting questions:
What is blunt cardiac injury and how is it diagnosed?
What are the potential complications and how should they be addressed?
Do all patients with chest trauma need an ECG? Troponin?
What about isolated sternal fractures?
What findings merit an emergent echo?
When should an otherwise well appearing patient be admitted for observation?
Overview of Blunt Cardiac Injury (BCI)
Blunt Cardiac Injury (BCI) encompasses a spectrum of disease caused by significant blunt force transmitted to the heart via a deceleration injury or direct blow to the precordium. Damage is done as a result of direct compression of the heart between the sternum and spine, increased intra-thoracic pressure, deceleration forces (the heart has relatively unrestricted movement in the AP direction so abrupt deceleration can cause a significant impact with the sternum), or direct trauma from fractured ribs1
BCI is an umbrella term that includes a spectrum of potential pathology such as:
Comotio Cordis: sudden death due to an ill-timed force during a period of electrical vulnerability
Cardiac rupture: traumatic rupture of the myocardium due to compression of a full chamber during early systole or raid deceleration forces shearing the atria from the vena cava or pulmonary veins.[1] Often identified on autopsy due to roughly 90% fatality within minutes
Pericardial rupture and cardiac herniation: very rare. Most likely will either result in death before arrival or will not be the direct cause of death.[1]
Valvular injury: laceration of aortic cusps can cause aortic insufficiency. Compression of heart during systole can lead to tearing of mitral valves and/or papillary muscle rupture.
Septal tear: traumatic ASD or VSD are less common pathological findings identifiable by characteristic loud holosystolic murmurs and echocardiography
Coronary artery dissection/thrombosis: rare to occur in isolation
Myocardial contusion: edema and necrosis of cardiac myocytes due to blunt traumatic injury
Of the above injuries, most are relatively easy to diagnosis. Comotio cordis, by definition, is not survivable. Cardiac rupture leads to immediate death in most cases, but if a stable hematoma forms, the patient may present alive and in tamponade, which can be identified clinically and with the aid of bedside ultrasound. Isolated pericardial rupture is very rare. It can be associated with cardiac herniation and subsequent impairment in cardiac output, which will manifest with unstable vitals or could be identified on echo. Valvular or septal injuries will often present with heart failure, and most will be associated with a loud, new murmur and/or hemodynamic instability. Coronary artery dissection is exceedingly rare, but diagnosis (ECG, troponin) and treatment (cardiology consultation, PCI) are similar to regular MI and not unfamiliar to the emergency physician. That leaves myocardial contusion, which is the subject of considerable debate and will be discussed in detail below.
There is no clear-cut definition or gold standard diagnosis for myocardial contusion. Pathologically, a cardiac contusion involves edema and necrosis of myocytes as well as patchy areas of hemorrhage, similar to that seen with an MI. Hence, cardiac troponins are very specific for myocardial injury from trauma just as they are for ischemic damage.[2] Serum levels are elevated much more rapidly than after MI, however some sources recommend a 4-6 hr delta troponin depending on time of initial presentation and level of suspicion.[2,3] However, cardiac contusions can occur in the absence of troponin elevation and can be variably diagnosed via TTE, TEE, or ECG. Although frequently encountered in high-risk poly-trauma patients, the vast majority of cardiac contusions tend to improve spontaneously and will heal with scar formation. They are generally well tolerated and may produce only minimal symptoms.[2] Prognosis is excellent both in-hospital and at 3 and 12 month follow up and patients who are initially clinically stable are very unlikely to deteriorate due to cardiac contusion.[4] There are two mechanisms by which blunt cardiac injury can lead to significant morbidity and mortality: significant contractile dysfunction and arrhythmia.
Significant contractile dysfunction is easy to identify by assessing the patient’s vital signs. A hemodynamically stable, asymptomatic patient is unlikely to be suffering from serious traumatic heart failure. Conversely, patients with hemodynamic instability or persistent arrhythmia should have an emergent echocardiogram to assess for a structural abnormality or hemodynamically significant contusion.[3]
Arrhythmia may have a delayed presentation in an otherwise asymptomatic patient. Therefore, “at risk” patients may benefit from a telemetry admission in order to identify and treat expeditiously. Twenty four hours is an appropriate duration for monitoring because evidence suggests that arrhythmia will almost always manifest within the first 24 hours.[2,5] To screen for those at risk, the Eastern Association for the Surgery of Trauma (EAST) guidelines strongly recommend an ECG on all patients with a potential mechanism.[3] Common mechanisms include motor vehicle collisions, falls from height, and crush injuries. In terms of defining a high-risk mechanism, the EAST guidelines are not specific, but many individual institutions specify particular speeds or characteristics of MVC or particular heights of falls that merit screening for BCI.
Of note, while an isolated sternal fracture is clearly indicative of significant force transmitted to the thoracic cavity, is should be thought of as a risk factor for BCI rather than pathognomonic. Only a small percentage of patients with isolated sternal fracture wind up with a cardiac contusion.[6] Hence, patients with sternal fracture should be screened (with an ECG and troponin as discussed above), but should not be immediately labeled with a diagnosis of myocardial contusion or blunt cardiac injury.
Prior guidelines hedged on the utility of a troponin, but the new 2012 EAST guidelines acknowledge several recent studies which have shown that a normal ECG alone may not be sufficient to rule out clinically significant BCI and that the addition of a negative troponin increases negative predictive value to 100%. Patients with a normal ECG and negative troponin can be ruled out for BCI.[3] This guideline is partially based on a prospective study, which evaluated 333 patients with significant thoracic trauma and concluded that patients with a normal ECG and a negative delta troponin (at 0 and 8 hrs) could be safely discharged if they lacked other criteria for admission.[7] Patients with either an ECG abnormality (arrhythmia, ST changes or evidence of ischemia, heart block) or an elevated troponin should be admitted for telemetry monitoring for 24 hours.[3]
Case Resolution
Our patient from above was admitted for 24 hour monitoring given his abnormal initial ECG. In addition to pain control, incentive spirometry, and supportive care for his rib fractures, he was monitored on telemetry given his elevated risk of dysrhythmia from a likely cardiac contusion. Fortunately, he had an uneventful hospital stay, repeat ECG showed resolution of the prior bundle branch block, and he was discharged the following afternoon.
Summary
Blunt cardiac injury (BCI) is an umbrella term encompassing a wide spectrum of pathology due to blunt thoracic trauma.
Hemodynamically unstable patients should receive an emergent echo. This will help to identify structural abnormalities such as cardiac, septal, or pericardial rupture, valvular disruption, or hemodynamically significant cardiac contusion.
At-risk patients should be screened with an ECG and a troponin. If both are normal, then clinically significant BCI is unlikely.
Isolated sternal fracture is a risk factor for BCI and should prompt screening with ECG and troponin, but is not pathognomonic and does not mandate additional BCI workup on its own
Patients with an abnormal ECG or an elevated troponin should be admitted for telemetry monitoring for 24 hours to ensure timely treatment if the patient develops a dysrhythmia.
Expert Commentary
Excellent overview of a broad and complicated topic; just a few points to clarify/emphasize. As you stated, blunt cardiac injury is truly a spectrum of injuries related to the delivery of significant force to the precordium/chest wall. For the most part, these patients are either stable or nearly dead. The truly serious injuries, such as comotio cordis or free cardiac/pericardial rupture, are generally fatal prior to hospital arrival. Blunt valvular injury tends to be hemodynamically significant and should be suspected in a patient with signs of cardiogenic shock or murmur. While the overall incidence of BCI in the setting of thoracic trauma ranges from 13-76%, it is rare to have serious complications from BCI, and most patients who are alive on arrival to the hospital have minor cardiac injuries. These are usually myocardial contusions or dysrhythmias, and tend to be asymptomatic and self-resolve within 24 hours.
Given the high incidence of BCI and poor sensitivity of physical exam, all patients with an appropriate mechanism should be screened with EKG and troponin. A normal EKG and negative troponin is sufficient to rule OUT blunt cardiac injury. Patients with EKG changes and/or positive troponins should be stratified by hemodynamics and clinical stability. Stable patients should be observed with telemetry for resolution of EKG changes, with serial EKG and troponins depending on the degree of abnormality. Any unstable patient with risk factors for BCI should undergo emergent echocardiography to identify a possible serious injury that would require intervention.
As a final note, the risk factors for BCI are also risk factors for aortic injury, so make sure to evaluate the aorta in unstable or symptomatic patients.
Emily Koeck, MD
Surgical Critical Care, Trauma, and Burn Fellow, John H. Stroger, Jr. Hospital of Cook County
How To Cite This Post
[Peer-Reviewed, Web Publication] Trinquero P, Gappmaier V (2018, September 10). Blunt Cardiac Injury. [NUEM Blog. Expert Commentary by Koeck E]. Retrieved from http://www.nuemblog.com/blog/BCI
Posts You May Also Enjoy
References:
El-Menyar A, Al Thani H, Zarour A, Latifi R. Understanding traumatic blunt cardiac injury. Ann Card Anaesth. 2012;15(4):287-295.
Sybrandy KC, Cramer MJ, Burgersdijk C. Diagnosing cardiac contusion: old wisdom and new insights. Heart. 2003;89(5):485-489.
Clancy K, Velopulos C, Bilaniuk JW, et al. Screening for blunt cardiac injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S301-306.
Lindstaedt M, Germing A, Lawo T, et al. Acute and long-term clinical significance of myocardial contusion following blunt thoracic trauma: results of a prospective study. J Trauma. 2002;52(3):479-485.
Fabian TC, Cicala RS, Croce MA, et al. A prospective evaluation of myocardial contusion: correlation of significant arrhythmias and cardiac output with CPK-MB measurements. J Trauma. 1991;31(5):653-659; discussion 659-660.
Athanassiadi K, Gerazounis M, Moustardas M, Metaxas E. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26(10):1243-1246.
Velmahos GC, Karaiskakis M, Salim A, et al. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma. 2003;54(1):45-50; discussion 50-41.
Understanding Inhalation Injury
Smoke inhalation injuries are one of the leading causes of morbidity and mortality in the United States. In emergency medicine, we must be aware of harbingers of impending airway issues that come along with this deadly injury. We discuss pathophysiology, diagnosis and management in this week's post.













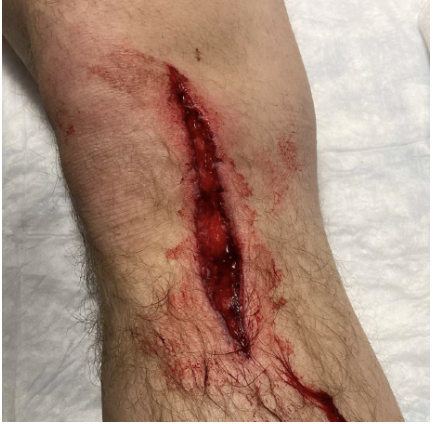








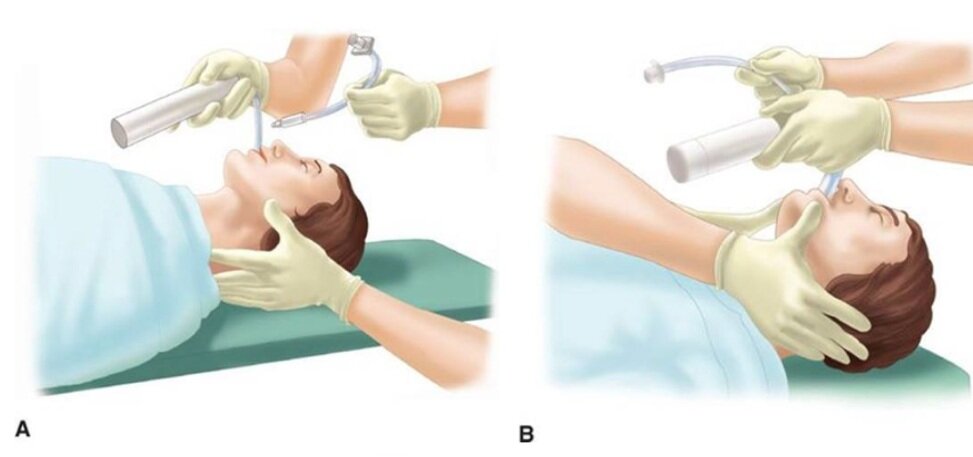






































![Figure 1: Bruising patterns that suggest child abuse. [6]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459114532-IVTA7KT3PX1J6DGC3U3L/Figure+1%3A+Patterns+of+Bruising)
![Figure 2: Forced immersion burn of buttocks with bilateral, symmetric leg involvement in a “stocking” pattern. [7]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459322548-EFHGSFQ962QP9FU5DNJN/Figure+2%3A+Burn+patterns+that+suggest+non-accidental+trauma)
![Figure 3: Classic metaphyseal lesion. White arrows denote femoral metaphyseal separation and black arrow denotes a proximal tibial lesion or “bucket handle.” [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459580501-8461RMF0P9706GJW7XJU/Figure+3%3A+Fractures+of+NAT)
![Figure 4: Posterior and lateral rib fractures of differing ages indicative of NAT [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460464292-4QFTGMDQAB64NQBSYD9N/Figure+4%3A++Rib+fractures+of+differing+ages+indicative+of+NAT)
![Figure 5: Fundus of child with AHT with too-numerous-to-count retinal hemorrhages indicated by the black arrows. [8] The white arrow indicates small pre-retinal hemorrhages. The white arrowhead denotes hemorrhage extending into the peripheral retina…](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460641270-3V7M52RMMV1QDMZ3PA1S/Figure+5%3A+Occular+manifestations)
![Figure 6: Elements of the Skeletal Survey. Although a full skeletal survey is currently the standard of care for patients with NAT, there are ongoing research efforts to tailor X-ray imaging more specifically to each patient. [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570461092995-EBKSMB5DI0GTGXY0R75N/Figure+6%3A+Skeletal+surgery)