Written by: Richmond Castillo, MD (NUEM ‘23) Edited by: Shawn Luo, MD (NUEM ‘22)
Expert Commentary by: Jacob Stelter, MD (NUEM ‘19)
Expert Commentary
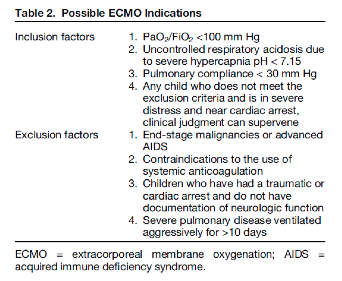
This is an excellent summary of the diagnosis and management of radial head subluxation (nursemaid’s elbow) in children. Clinically, as pointed out, these patients are usually toddlers and will come in after an injury to the arm. Usually, the clinical history will involve the child’s arm having been pulled on while the elbow was extended leading to sudden onset of pain and reduced mobility of the arm. The patient will most often be holding the elbow in flexion and be resistant to having it manipulated. In general, I have a low threshold to obtain radiographs on these patients. If the story and exam is classic for a radial head subluxation, imaging is technically not indicated, and reduction can be attempted. However, more often than not, the history can be vague, and the mechanism of injury may be unclear. In this situation, it is better to rule out a fracture first than to attempt a reduction without imaging. Attempted reduction could worsen or lead to displacement of a supracondylar humerus fracture if that is present. Keep in mind that it is not uncommon for the subluxation to reduce spontaneously during the process of obtaining x-rays.
There are two preferred techniques for reduction of a radial head subluxation. The method I start with is to support the patient's elbow and forearm and gently supinate the forearm while flexing the elbow and applying gentle pressure over the radial head. A “pop” sensation will often be felt as the radial head reduces. The other technique that can be used is to hyper-pronate the forearm while maintaining the elbow in a flexed position. Both of these techniques have a high success rate. Typically, the child will start using the arm again, but it may not be immediate. I will typically reassess the patient about 10-15 minutes post-reduction to ensure they are using their arm normally again. If the child is using their arm and able to extend and flex at the elbow without pain, they can be discharged, and no splinting is necessary. If no radiographs were obtained prior to reduction and the patient is not back to baseline post-reduction, x-rays should be obtained to rule out a fracture. Keep a broad differential, especially if the patient is not responding as you would expect or has other vital sign or exam abnormalities.
Jacob Stelter, MD, CAQ-SM
Division of Emergency Medicine | NorthShore University HealthSystem
NorthShore Orthopaedic Institute | Primary Care Sports Medicine
Clinical Assistant Professor | University of Chicago Pritzker School of Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Castillo, R. Luo, F. (2022, Feb 7). Nursemaid’s Elbow. [NUEM Blog. Expert Commentary by Stelter, J]. Retrieved from http://www.nuemblog.com/blog/nursemaids-elbow.



































![Figure 1: Bruising patterns that suggest child abuse. [6]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459114532-IVTA7KT3PX1J6DGC3U3L/Figure+1%3A+Patterns+of+Bruising)
![Figure 2: Forced immersion burn of buttocks with bilateral, symmetric leg involvement in a “stocking” pattern. [7]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459322548-EFHGSFQ962QP9FU5DNJN/Figure+2%3A+Burn+patterns+that+suggest+non-accidental+trauma)
![Figure 3: Classic metaphyseal lesion. White arrows denote femoral metaphyseal separation and black arrow denotes a proximal tibial lesion or “bucket handle.” [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459580501-8461RMF0P9706GJW7XJU/Figure+3%3A+Fractures+of+NAT)
![Figure 4: Posterior and lateral rib fractures of differing ages indicative of NAT [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460464292-4QFTGMDQAB64NQBSYD9N/Figure+4%3A++Rib+fractures+of+differing+ages+indicative+of+NAT)
![Figure 5: Fundus of child with AHT with too-numerous-to-count retinal hemorrhages indicated by the black arrows. [8] The white arrow indicates small pre-retinal hemorrhages. The white arrowhead denotes hemorrhage extending into the peripheral retina…](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460641270-3V7M52RMMV1QDMZ3PA1S/Figure+5%3A+Occular+manifestations)
![Figure 6: Elements of the Skeletal Survey. Although a full skeletal survey is currently the standard of care for patients with NAT, there are ongoing research efforts to tailor X-ray imaging more specifically to each patient. [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570461092995-EBKSMB5DI0GTGXY0R75N/Figure+6%3A+Skeletal+surgery)