Written by: Keith Hemmert, MD (EM Resident Physician, PGY-3, NUEM); Edited by: Michael Macias, MD (EM Resident Physician, PGY-4, NUEM); Expert Commentary by: Michael Gisondi, MD
The Case
A middle-aged gentleman with a history of hypertension presented to the emergency department via EMS with altered mental status.
The pre-hospital report was ‘syncope vs seizure.’ On arrival, the patient was lethargic but rousable to voice, and only able to answer yes/no questions. He became obtunded within minutes.
His initial vitals were notable for hypertension with a systolic blood pressure greater than 220 mm Hg. He was emergently intubated for airway protection in the setting of rapidly deteriorating mental status. Prior to intubation, he was noted to have a disconjugate gaze.
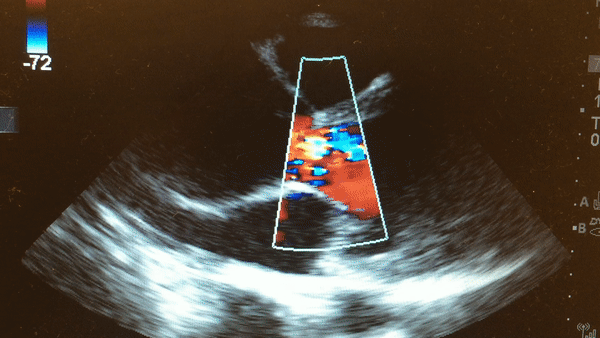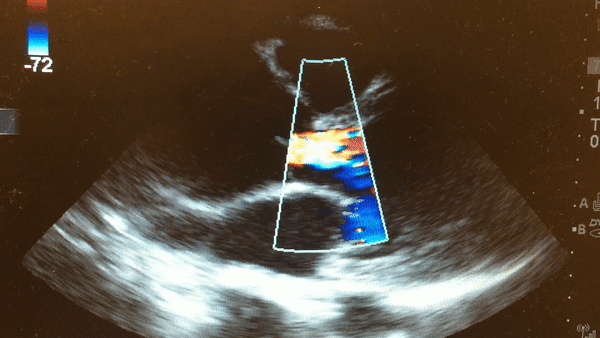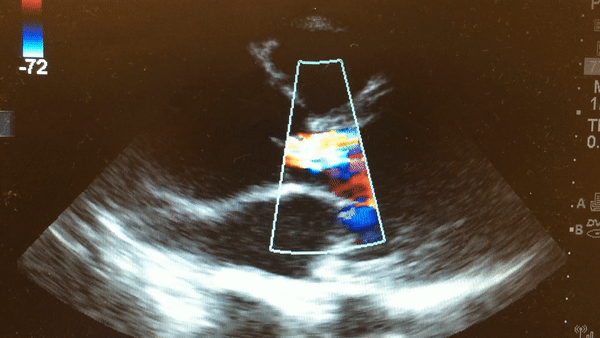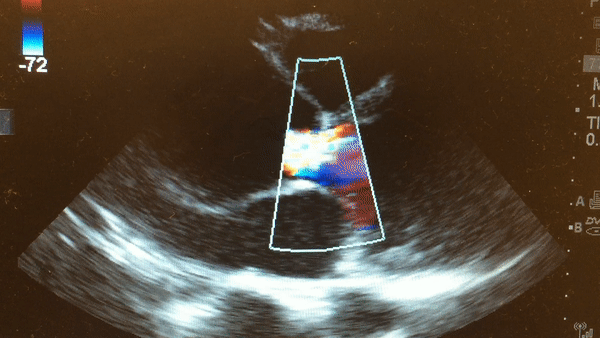
Once intubated and paralyzed, an ECG was obtained that demonstrated NSR with no ischemic changes. While preparing the patient for transport to the CT scanner, a bedside echo was performed that demonstrated the following:
This is a parasternal long axis view demonstrating significant enlargement of the aortic root with severe aortic regurgitation.
Given the dilated aortic root, our suspicion for dissection increased significantly. Images from his CT brain and CT chest/abdomen/pelvis revealed the following:
No acute intracranial process was noted on non-contrast CT of the brain. CT aortogram revealed a thoracic aortic dissection with an aortic root dilated to 5cm, a dissection flap throughout the ascending aorta, descending thoracic aorta, and throughout the entire abdominal aorta, extending into both iliac artery. The dissection further involved both carotid arteries, the left renal artery, and the celiac trunk.
Vascular surgery was consulted and aggressive resuscitation measures were initiated including placement of an arterial line and central venous catheter, and initiation of an esmolol drip to maintain systolic blood pressure < 140mm Hg. The patient survived the operating room but expired several days later in the ICU.
Discussion
Aortic dissection is described in Tintinalli’s as occurring “after a violation of the intima allows blood to enter the media and dissect between the intimal and adventitial layers.” [1] This forms a false lumen that is essentially a dissecting column of blood. It may re-dissect back into the intima, or it may dissect through the adventitia, which is usually fatal.
This entity is uncommon (~5-30 per 1 million people per year) and typically seen in the setting of chronic hypertension, as well as certain systemic disease such as bicuspid aortic valve, Marfan Syndrome, or Ehlers-Danlos Syndrome. While in this case, our patient presented with marked hypertension, it is chronic hypertension that truly puts patients at risk for this disease. Up to 1 in 4 patients with Stanford Type A dissection will have a presenting systolic blood pressure below 100mm Hg. [2]
Classically it is taught that a dissection presents as a “ripping or tearing” pain going to the back. An article by Hagan et al has a remarkable case series of 464 dissections, including descriptors of the pain, that is worth reviewing. Of note, while over 90% of patients felt that it was the worst pain they had ever experienced, only 50% of subjects described their pain as ripping or tearing (62% described pain as sharp), only 35% had any posterior chest pain, and only 85.4% of patients described the onset of their pain as ‘acute.’
The varied presentation of the disease makes aortic dissection difficult to diagnose, and the clinician should have a high index of suspicion for this life-threatening disease process.
Typical exam findings in aortic dissection include an aortic insufficiency murmur, a pulse deficit in radial or femoral arteries, and a difference between right sided and left sided blood pressures. Unfortunately, chest radiographs have poor sensitivity for detecting aortic dissection; 37.4% of patients with a Type A Dissection had a normal mediastinal appearance in one study.[2] A provider can examine the aortic root with bedside ultrasound, as we did in this case, to evaluate aortic root diameter, assess for complications such as pericardial effusion, or attempt to visualize a dissection flap in the aorta. Definitive diagnosis is made CT aortography or angiogram.
Management Principles of Aortic Dissection
In the emergency department, the management of aortic dissection centers around heart rate and blood pressure control, with the goal of reducing the blood pressure to the lowest possible that maintains adequate end organ perfusion. A good target to aim for is < 120 mmHg. In type A aortic dissection, blood pressures in both arms can be unequal, so you should target your therapy to modify the higher blood pressure reading obtained. The preferred intravenous agents for achieving blood pressure control is esmolol, given its favorable pharmacokinetic properties (quick on/off). Labetalol can also be used. If there is a contraindication to beta blocker use, diltiazem or verapamil should be substituted.
The physiological goal of blood pressure control is to decrease continued shear stress on the dissection flap and prevent further damage; to achieve this goal, a decrease in blood pressure as well as a decrease in chronotropy are vital.
The conventional treatment for Type A dissections is surgical management, whereas Type B dissections typically receive medical management. Newer endovascular therapies have gained acceptance as treatment for some type A and type B dissections.
This case was notable for the lack of history available to the provider, complicating the differential diagnosis. It serves as an important reminder that aortic dissection must remain in the clinician’s differential for an unresponsive patient.
Take Home Points
- Acute aortic dissection is an uncommon, highly morbid disease.
- Complications can develop rapidly and the outcome is often fatal without early intervention.
- Chronic hypertension is a predominate risk factor for dissection.
- The typical presentation of dissection is characterized by acute onset of severe pain with key descriptors being “sharp, ripping, tearing, migrating, or radiating,” however classic signs and symptoms of aortic dissection are often absent and a high clinical index of suspicion is necessary.
- Ultrasound is an important bedside adjunct in patients presenting with suspected acute aortic dissection.
- Management of aortic dissection in the emergency department should focus on blood pressure/heart rate reduction to reduce shear stress on the dissection flap, pain control, and vascular surgery consultation.
Expert Commentary: “Outliers”
Thank you for the opportunity to comment on this remarkable case. Kudos to the emergency provider who considered this important diagnosis in their differential and used their bedside ultrasound skills to confirm their suspicion. (Jedi-like!) VERY IMPRESSIVE, for several reasons: aortic dissection is rarely mentioned on the differential of acute mental status change (as dissection flaps do not always affect cranial blood flow); the ultrasound images in this case are difficult to obtain and represent an advanced provider skill set; and the usual pace of activity in a resuscitation, such as can be imagined in this case, does not normally lend itself to obtaining quick, high-quality, challenging ultrasound images. For all these reasons, I salute the resident in this case for their amazing clinical acumen and diagnostic ability. A true, outlier.
In his book, Outliers, Malcolm Gladwell asks the question, “what makes high-achievers different?” For the provider in this case, you might agree that they a high-achiever for the obvious reasons that I list above: an unusually thoughtful differential + advanced ultrasound skills + the ability to elbow others out of the way to perform the ultrasound amidst a resuscitation. To paint with broader strokes, this represents uncommon management of an uncommon disease that has an uncommon presentation.
Aortic dissection is itself, an outlier. The incidence of aortic dissection, somewhere on the order of 1 in 100,000 to 1 in a 1,000,000, makes it an uncommon disease (With that low incidence, it is shocking that so many outliers in our society have died from aortic dissection, such as Lucille Ball, King George II, James Holbrook, John Ritter and Alan Thicke).
Emergency physicians pride themselves on considering uncommon disease in our differentials. The sky is always falling unless we prove otherwise, right? Every day in our emergency department there are dozens of patients who present with chest pain and, for each of those patients, we consider the six causes of chest pain with the highest rates of mortality in 24 hours: acute coronary syndrome, tension pneumothorax, pulmonary embolism, cardiac tamponade, esophageal rupture, and… aortic dissection. Several of those are uncommon diseases, but we must consider them all for each of our chest pain patients because all six diagnoses are potentially treatable in the acute setting.
The problem isn’t that we forget to consider aortic dissection, but rather that we forget that aortic dissection has an uncommon presentation. Yes, we all learn early in medical school that aortic dissection has the ‘classic’ presentation of ripping chest pain radiating to the back in a patient with high blood pressure. But for this disease, ‘classic’ does not mean ‘common.’ Instead, I would challenge you to remember that aortic dissection often presents with significant variations of the ‘classic’ presentation: an uncommon presentation pattern, if you will.
As in the resident’s post above, one large study found that 2/3 of patients have sharp rather than tearing pain, 2/3 of patients have no radiation of their pain to the back, and almost 1 out of 5 patients have subacute pain. There are numerous studies in the radiology literature that document the uncommon presentation of ‘classic’ chest x-ray findings, most of which are found retrospectively after a CT chest is performed. Some (but not all) patients have neurological complaints, some (but not all) patients have abnormal peripheral blood flow to at least one limb… you see where I am going with this. Over my 15 years of practice, I think the variations of the ‘classic’ presentation – or the uncommon presentation – is the norm.
Here are a few ways to overcome the uncommon presentation issue:
Chest pain + high blood pressure…#thinkaorticdissection.
Chest pain + low blood pressure…#thinkaorticdissection.
Chest pain + abnormal pulses…#thinkaorticdissection.
Chest pain + neurological symptoms…#thinkaorticdissection.
Severe chest pain + poor response to pain meds…#thinkaorticdissection.
Given the low incidence of this uncommon disease, it is worth crowd-sourcing some additional decision-making hacks. I welcome readers to comment below and/or take the discussion to Twitter for some additional pearls using #thinkaorticdissection #nuemblog.
Again, thanks for the opportunity to provide a few thoughts on outliers and aortic dissection.
Mike Gisondi, MD (Twitter: @MikeGisondi)
Vice Chair of Education
Department of Emergency Medicine, Stanford University
Other Posts You May Enjoy
How To Cite This Blog Post
[Peer-Reviewed, Web Publication] Hemmert K, Macias M (2017, May 30). Aortic Dissection: Practice Update. [NUEM Blog. Expert Commentary By Gisondi M]. Retrieved from http://www.nuemblog.com/blog/aortic-dissection
References
- Johnson GA, Prince LA. Chapter 59: Aortic Dissection and Related Aortic Syndromes Tintinalli’s Emergency Medicine: A Comprehensive Review (8e).
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): New Insights Into an Old Disease. JAMA. 2000;283(7):897-903. doi:10.1001/jama.283.7.897.
- Hiratzka LF, Bakris GL, Beckman JA, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 121: e266–369.
- Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular Repair of Type B Aortic Dissection: Long-term Results of the Randomized Investigation of Stent Grafts in Aortic Dissection Trial. Circ Cardiovasc Genet. 2013 Aug 1. 6(4):407-16.
- Shu C, Wang T, Li QM, Li M, Jiang XH, Luo MY, et al. Thoracic endovascular aortic repair for retrograde type A aortic dissection with an entry tear in the descending aorta. J Vasc Interv Radiol. 2012 Apr. 23(4):453-60, 460.e1.
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999 May 20. 340(20):1546-52.
- Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999 May 20. 340(20):1539-45.