Written by: Trish O’Connell, MD (NUEM ‘22) Edited by: Will Ford (NUEM ‘19) Expert Commentary by: Dion Tyler, PharmD and Bayan Al-Namnakani, PharmD
Expert Commentary
IM olanzapine + IM/IV benzodiazepines: The FDA-approved package insert for olanzapine currently recommends general avoidance of intramuscular olanzapine and parenteral benzodiazepines [1]. The European Medicines Agency (EMA) recommends waiting > 1 hour following IM olanzapine administration to administer benzodiazepines with careful monitoring for excessive sedation and cardiorespiratory depression, likely taking into account the 15-45 minute time to reach peak concentrations for IM olanzapine [2].
The warning against coadministration of intramuscular olanzapine and benzodiazepines (BZDs) arose from postmarketing data of adverse events in patients receiving intramuscular olanzapine for acute agitation [3]. This study reported that BZD use was associated with 51.7% (15/29) of fatal cases and 85.7% (24/28) of serious adverse drug reactions, defined as those that were life-threatening, extended the hospital stay, or resulted in a permanent disability. The authors thus recommended that the combination of IM olanzapine and benzodiazepines be avoided in the absence of further prospective data. However, it is important to note that many of these patients in this cohort had severe comorbidities, and BZD association included all instances (oral, IV, or IM) of BZD administration throughout their hospital stay. Some patients expired several days or weeks following their last olanzapine dose, making a causal association difficult to determine. Subsequent, smaller cohorts have found that oxygen desaturations are greatest in individuals who receive olanzapine and BZD therapy and have consumed ethanol, yet desaturation rates were similar with this combination in patients without ethanol intoxication when compared to olanzapine and haloperidol monotherapy as well as haloperidol and BZD combination therapy [4,5]. A retrospective, medication use evaluation (MUE) of IM olanzapine and lorazepam also demonstrated no incidences of hypotension or oxygen desaturation when the combination was administered within 1-hour or 24-hours of each other [6]. Lastly, a prospective cohort of individuals receiving IV olanzapine or IV droperidol followed immediately by IV midazolam compared with IV midazolam alone also demonstrated similar rates of oxygen desaturations and adverse events among all three groups [7]. However, IV olanzapine is not approved for use in acute agitation, and may display different pharmacokinetics compared to IM administration.
While evidence supporting the safe use of this combination is growing, it may be prudent to use caution while coadministering IM olanzapine and BZDs in the absence of further controlled studies and in patients at greatest risk for adverse events, including the elderly and those who’ve consumed ethanol.
References:
Olanzapine [package insert]. Indianapolis, IN: Eli Lilly and Company, 2010.
Zyprexa. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/zyprexa. Accessed June 28, 2020.
Marder SR, Sorsaburu S, Dunayevich E, et a.l. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-41.
Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olaznapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-96.
Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-97.
Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin. 2018;8(5):208-13.
Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61:72-81.
Myasthenia gravis and medications in the emergency department: Myasthenia gravis (MG) is an autoimmune disorder resulting in destruction of acetylcholine receptors at the neuromuscular junction (NMJ) and resultant muscular weakness. While not included in the original blog post, this is a drug-disease interaction where pharmacy services are frequently recruited for assistance in choosing medications that will not cause or exacerbate a myasthenic crisis. Although not all-inclusive, common agents that may be frequently encountered in the ED are listed below [1-3]:
Neuromuscular blocking agents (paralytics): Succinylcholine exerts its therapeutic effects through depolarization of the acetylcholine receptor at the NMJ causing sustained paralysis. In the setting of MG and reduced acetylcholine receptors, succinylcholine requirements may be increased, necessitating a higher dose of 1.5-2 mg/kg. Conversely, MG patients are more sensitive to nondepolarizing neuromuscular blockers,such as rocuronium and vecuronium, requiring a lower dose than normal. For rocuronium, a dose of 0.3-0.6 mg/kg may be considered for these patients.
Antibiotics: Several classes of antibiotics have been shown to prevent transmission of acetylcholine to the acetylcholine receptor at varying levels of risk listed below:
High risk: aminoglycosides, fluoroquinolones
Medium risk: Macrolides, polymixin B
Low risk: Penicillins, cephalosporins, carbapenems, nitrofurantoin, clindamycin, sulfonamides, doxycycline
Magnesium: Magnesium interferes with release of acetylcholine to the NMJ and may exacerbate a myasthenic crisis. A higher threshold for repletion may be necessary in MG patients as well as avoidance of use for migraines, tachyarrhythmias, and as a component of laxatives.
Beta blockers: Beta blockers also appear to have an effect at the NMJ in preventing acetylcholine transmission, and have been found to exacerbate MG symptoms in patients with a variety of different agents in the class and routes of administration, such as ophthalmic timolol.
Corticosteroids: While frequently used as treatment for a MG crisis, these agents may paradoxically worsen muscle strength through acetylcholine receptor blocking and effects on muscle contractility.
References:
Roper J, Fleming ME, Long B, et al. Myasthenia gravis and crisis: evaluation and management in the emergency department. J of Emerg Med. 2017;53:843-53.
Ahmed A, Simmons Z. Drugs which may exacerbate of induce myasthenia gravis: a clinician’s guide. The Internet Journal of Neurology. 2008;10:e1-8.
Singh P, Idowu O, Malik I, et al. Acute respiratory failure induced by magnesium replacement in a 62-year-old woman with myasthenia gravis. Tex Heart Inst J. 2015;42(5):495-97.
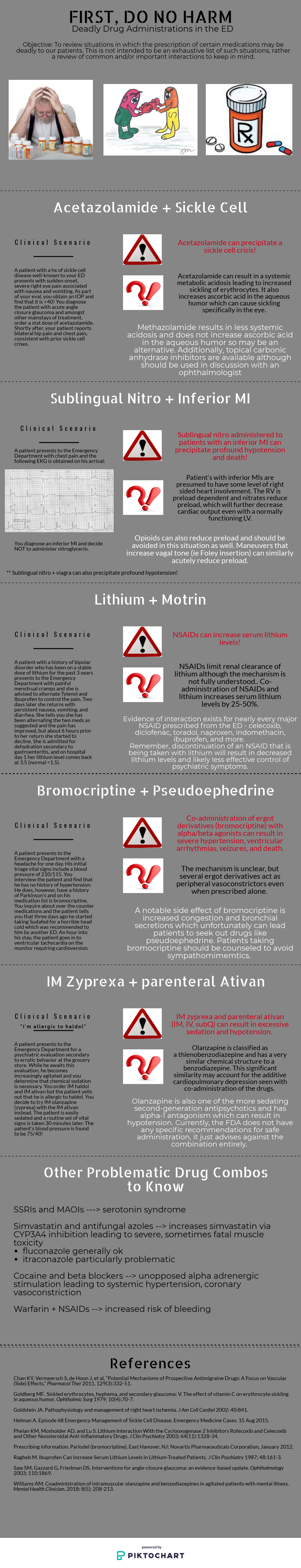
Lithium + ibuprofen (NSAIDs): Treatment with lithium could be quite challenging due to its extremely narrow-therapeutic index (0.5–1.2 mEq/L). Therefore, minor changes affect serum levels. The most common lithium poisoning occurs unintentionally (with chronic use) when the lithium intake exceeds its elimination such as in impaired kidney function or due to drug-drug interaction.1
Lithium is a water-soluble monovalent cation widely distributed in the body and it goes complete glomerulus filtration, 75 % of the ion is reabsorbed mainly in the proximal tubule. The exact mechanism is not fully understood. It appears that NSAIDs decrease the eGFR resulting in decreased lithium renal excretion. Some experts hypothesized that this is a result of the prostaglandin synthesis inhibition by NSAIDs which may lead to low renal blood flow and facilitate the reabsorption of sodium and lithium (theoretically). However, this hasn’t been proven [1].
This interaction is well known in clinical practice and most providers will be cautious when it comes to NSAIDs. Small prospective studies have shown large interindividual differences in lithium clearance associated with different NSAIDs. Those effects are highly variable and less predictable. It can occur with any NSAID and studies haven’t concluded a strong relationship with a specific agent. They have reported a reduction in lithium level by 10-25% in healthy volunteers [1-2], and up to 60% in another study [3]. A small retrospective study quantified the relative risk of lithium toxicity secondary to a medication new start in elderly patients who are on lithium. The relative risk was dramatically higher with ACEIs (RR=7.6, 95% CI=2.6–22.0) and loop diuretics (RR=5.5, 95% CI=1.9–16.1). Interestingly, NSAIDs and thiazides were not independently associated with increased risk of lithium toxicity [5].
Lithium levels and toxic effects should be monitored with concomitant NSAIDs initiation. Consider lithium dose reduction especially with NSAIDs new start or dose increase.
References:
Finley, P.R. Drug Interactions with Lithium: An Update. Clin Pharmacokinet 55, 925–941 (2016).
Reimann IW, Diener U, Frölich JC. Indomethacin but not aspirin increases plasma lithium ion levels. Arch Gen Psychiatry. 1983;40(3):283–6.
Ragheb MA. Aspirin does not significantly affect patients’ serum lithium levels. J Clin Psychiatry. 1987;48(10):425.
Ragheb M. Ibuprofen can increase serum lithium level in lithium-treated patients. J Clin Psychiatry. 1987;48(4):161–3.
Juurlink, D.N., Mamdani, M.M., Kopp, A., Rochon, P.A., Shulman, K.I. and Redelmeier, D.A. (2004), Drug‐Induced Lithium Toxicity in the Elderly: A Population‐Based Study. Journal of the American Geriatrics Society, 52: 794-798. doi:10.1111/j.1532-5415.2004.52221.x.
Nitroglycerin (NTG) and inferior MI: Great job to the authors on describing an interaction that comes up quite frequently in the emergency department. The ACC/AHA guidelines on acute STEMI recommend avoidance of NTG in patients with RV dysfunction, pre-existing hypotension, marked bradycardia or tachycardia, and use of phosphodiesterase-5 inhibitor (PDE5) use in the previous 24-48 hours [1]. Specifically, 24 hours should elapse following sildenafil use, and 48 hours following tadalafil use, due to the difference in pharmacokinetics between these PDE5s [2,3].
It appears that this recommendation has been challenged by a retrospective analysis conducted by Robichaud and colleagues assessing the incidence of hypotension in prehospital patients with inferior STEMI and acute chest pain receiving NTG compared with those who did not receive NTG [4]. The determination of STEMI was made by a computer-interpreted electrocardiogram (ECG) utilized by EMS while in the prehospital setting. The researchers found similar rates of hypotension between the two groups, but stated that a computer-interpreted ECG cannot be used as the sole predictor for patients who may be predisposed to hypotension following NTG administration. In the absence of controlled data, it may be necessary to exercise caution when considering NTG in STEMI patients with known RV involvement and avoid use in hypotensive patients.
References:
O'Gara PT, Kushner FG., Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circ. 2013;127:e362-e425.
Sildenafil [package insert]. New York, NY: Pfizer Labs, 2014.
Tadalafil [package insert]. Indianapolis, IN: Eli Lilly and Company, 2018.
Robichaud L, Ross D, Proulx M, et al. Prehospital nitroglycerin safety in inferior ST elevation myocardial infarction, Prehosp Emerg Care. 2016;20(1):76-81.
Dion Tyler, PharmD
Emergency Medicine Pharmacy Specialist
Sinai Health System
Chicago, IL
Bayan Al-Namnakani, PharmD
PGY-2 Emergency Medicine Clinical Fellow
Northwestern Memorial Hospital Pharmacy
How To Cite This Post:
[Peer-Reviewed, Web Publication] O’Connell, T. Ford, W. (2020, Nov 29). Drug Interactions. [NUEM Blog. Expert Commentary by Tyler, D. Al-Namnakani, B]. Retrieved from http://www.nuemblog.com/blog/drug-interactions.