A case discussion and exploration of the mechanisms and complications of Kratom use
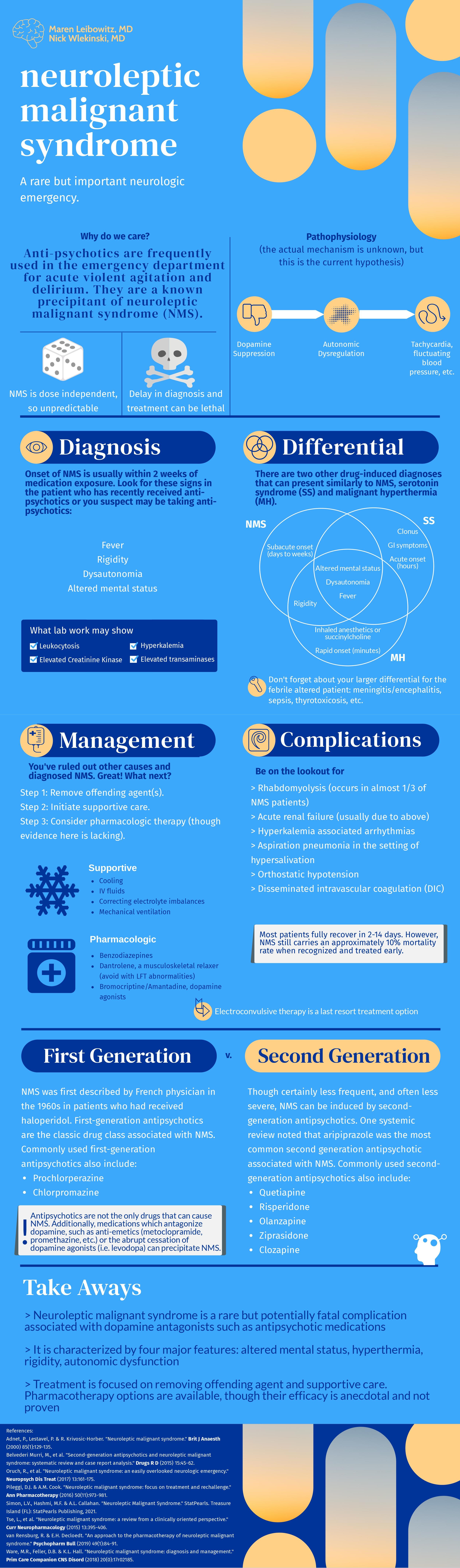
Neuroleptic Malignant Syndrome
Written by: Maren Leibowitz, MD (NUEM ‘23) Edited by: Nick Wleklinski, MD (NUEM ‘22)
Expert Commentary by: Zachary Schmitz, MD (NUEM '21)
Expert Commentary
This is an awesome, focused review of neuroleptic malignant syndrome (NMS). NMS is hard to diagnose because it's rare. There is no gold standard with respect to its definition, and it requires a medication history (which we typically don't do very well in the emergency department). A tricky cause of NMS is the removal of a dopamine agonist. For this reason, carbidopa/levodopa should never be discontinued during hospital admission - or ED boarding. [1]
Supportive care is more important than antidotal therapy during NMS management. The most acute cause of death from NMS is hyperthermia, which is induced both by D2 receptor antagonism leading to rigidity and impaired thermoregulation from the striatum and hypothalamus. Any life-threatening hyperthermia should be treated immediately with an ice bath.[2] Rigidity will lead to rhabdomyolysis with subsequent hyperkalemia and myoglobin-induced renal failure. Therefore, fluid resuscitation and maintenance are important. Profound immobility can precipitate DVT, so anticoagulation may be necessary.
In terms of pharmacotherapy, benzodiazepines are universally used. Dantrolene inhibits calcium-mediated muscle contraction to reduce muscle rigidity. However, it doesn't address the underlying central D2 antagonism, and its efficacy has only been shown in case reports. Bromocriptine acts more centrally as a dopamine agonist but should be used cautiously in patients with psychiatric diseases as it may exacerbate psychosis. Overall, benzodiazepine use and supportive care should get you through most cases of NMS, though additional therapies may be necessary in severe cases.
References
1. Institute for Safe Medication Practices. Delayed Administration and Contraindicated Drugs Place Hospitalized Parkinson’s Disease Patients at Risk. 12 March 2015. Accessed February 11, 2022.
2. Juurlink JN. Antipsychotics. In: Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS. eds. Goldfrank's Toxicologic Emergencies, 11e. Page 1037-1039. McGraw Hill; 2019. Accessed February 11, 2022.
Zachary Schmitz, MD
Toxicology Fellow
Ronald O. Perelman Department of Emergency Medicine
NYU Langone Health
How To Cite This Post:
[Peer-Reviewed, Web Publication] Leibowitz, M. Wleklinski, N. (2022, May 9). Neuroleptic Malignant Syndrome. [NUEM Blog. Expert Commentary by Schmitz, Z]. Retrieved from http://www.nuemblog.com/blog/neuroleptic-malignant-syndrome.
Other Posts You May Enjoy
Marine Envenomations
Written by: Michael Tandlich, MD (NUEM ‘24) Edited by: Chloe Renshaw, MD (NUEM ‘22)
Expert Commentary by: Justin Seltzer, MD (NUEM ‘21)
Expert Commentary
An excellent post by Drs. Tandlich and Renshaw. Marine envenomations are common problems around the world. Like with land-based envenomations, the venomous organisms of note vary with geography; jellyfish encountered in Australia are different from those encountered in Florida, for example. As a result, we will focus on major envenomations in the United States.
The invertebrates account for a large but ultimately unknown number of envenomations. Cnidaria includes jellyfish, hydrozoa, anemones, and fire coral. A majority of stings from this group result in painful dermatitis; tentacles create a “whip-like” pattern on the skin, whereas fire coral creates localized skin wheals. The sea nettle and Portuguese man-of-war are of greatest interest, given their potential to cause severe systemic symptoms. Box jellyfish are rare in US coastal waters but produce a life-threatening toxicity.
Initial treatment is somewhat controversial. Many resources advocate for the use of seawater for the initial decontamination, given concern for vinegar triggering nematocyst release in some species common to US waters. However, further research is needed to determine which is best. At this time, seawater is recommended for empiric decontamination in the US unless a box jellyfish is strongly suspected, in which case vinegar is appropriate (a very rare circumstance). Systemically ill box jellyfish envenomations should be treated with pain and blood pressure control. The antivenom is not readily available in the US and is unlikely to be beneficial in the time course it would take to obtain it.
Echinodermata, which includes sea urchins, have mild venom on their spines that can cause local tissue irritation and pain. There are reports of severe envenomations with systemic symptoms, but this is ultimately quite rare. These injuries respond well to hot water immersion. Imaging and local wound exploration for retained spines are recommended. Soaking the wound in vinegar may help dissolve superficial spines.
Of the vertebrates, stingrays and spiny fish are of primary concern.
Stingrays stings are common and can cause serious penetrating trauma but envenomation mainly produces localized pain and swelling. The venom is heat-labile, so significant pain relief can be achieved with hot water immersion. Stingrays stings have the potential for both retained stinger and wound infections; evaluation for retained stinger with radiographs and local wound exploration is recommended along with prophylactic antibiotics.
Spinyfish, in particular stonefish, lionfish, and scorpionfish, have venom located in their spines. Stonefish have the most potent venom of any known fish. Lionfish are not native to the US but have become an invasive species. Human contact with these fish occurs both in the wild and in aquariums. These fish also have heat-labile venom susceptible to hot water immersion. However, systemically ill stonefish envenomations should receive the antivenom as this envenomation can be life-threatening; the antivenom will likely work against other spiny fish too, however, these other envenomations are usually much less severe and rarely require more than hot water immersion and supportive care.
So key learning points:
Most marine envenomations involve heat-labile venom. Hot water immersion is likely to help reduce local symptoms.
Systemic illness is rare but some marine envenomations can produce life-threatening toxicity. Be very wary of a systemically ill envenomation and try to figure out the source due to the limited availability of antivenoms.
Prophylactic antibiotics are recommended for stingray stings as they tend to get infected but otherwise are generally not necessary in most populations. Good wound care, evaluation for retained foreign bodies, and tetanus prophylaxis are the mainstays.
For further information, see this review article
Justin Seltzer, MD
UCSD Health Toxicology Fellow
Emergency Physician, UCSD Health
How To Cite This Post:
[Peer-Reviewed, Web Publication] Tandlich, M. Renshaw, C. (2022, Mar 7). Marine Envenomations. [NUEM Blog. Expert Commentary by Seltzer, J]. Retrieved from http://www.nuemblog.com/blog/marine-envenomations
Other Posts You May Enjoy
Management of Snake Bite Injuries
Written by: Rafael Lima, MD (NUEM ‘23) Edited by: Mike Conrardy, MD (NUEM ‘21) Expert Commentary by: Sean Bryant, MD
An estimated 10,000 patients visit emergency departments for snake bite injuries each year in the United States [1]. The number of snake bite occurrences an emergency department sees depends largely on the geographic area of practice. While there are known remedies for these incidents, snake bites can be devastating if not promptly managed, meaning emergency physicians should be knowledgeable in the subject. In this article, we review the common management of snake bite injuries and envenomations for the two major snake groups in the United States.
Overview
There are about 20 known venomous species of snakes in the United States. While most envenomations occur in the Southwestern United States, every region is home to at least one species of venomous snake [2]. Not all snake bites result in envenomation. At least 25% of venomous snake bites are dry. You should still suspect envenomation upon the patient’s initial presentation and rule it out by monitoring their clinical symptoms and progression.
Identification of the snake is useful in guiding management of care, but it should not be attempted if doing so poses any additional risk to the patient or provider. In the United States, venomous snakes generally fall under two categories: Crotaline/pit vipers in the Viperidae family, and coral snakes in the Elapidae family.
Crotaline (Pit Vipers)
This group of snakes has historically been responsible for the more severe envenomations between the two groups [3]. The WHO classifies pit vipers in CAT 1 of their venom database, describing them as highly venomous with high rates of morbidity and mortality [4].
Pit vipers generally have a triangular shaped head with heat-sensing “pits” located on the face. They frequently have a rattle on their tail, but not all pit vipers are rattlesnakes. Copperheads and cottonmouth snakes are also included in this group.
Crotaline venom causes localized tissue necrosis and congestive coagulopathy. This can be identified by a prolonged INR, PT, PTT, and thrombocytopenia. Additionally, the viper venom can cause capillary and cellular membranes to increase in permeability. Large amounts of venom can cause diffuse vaso-extravasation and hemolysis that can lead to hypovolemic shock and DIC if untreated.
CroFab is the antivenom of choice for cotaline envenomation. It is a polyvalent antivenom, meaning it contains antibodies derived from the venom of multiple different species of snakes. Administration is titrated based on clinical and symptom response.
Elapidae
The venomous Elapidae snake in the United States is the coral snake. There are less severe envenomations from coral snakes compared to pit vipers. This is a result of how venom is administered between the two groups: pit vipers have venom glands that inject venom directly through the fangs, while coral snakes rely on passive seeping of venom through their glands while they chew.
Source: Tad Arensmeier from St. Louis, MO, USA
Coral snakes can be identified by their brightly colored rings extending along the length of the whole body. Usually, every other ring is yellow, separating the wider red or black rings in between. The common saying “red on yellow, kill a fellow; red on black, venom lack” has been been used to differentiate between venomous coral snakes and their harmless look-alikes in North America. A further level of differentiation is how far the rings extend circumferentially around the snake. Rings encircle the entire body in venomous coral snakes, while harmless look-alikes do not have the red coloration on the ventral side [5].
Source: Dawson at English Wikipedia
Venomous bites by coral snakes usually elicit little to no pain. This is because the Elapidae venom acts upon the neuromuscular junction and inhibits acetylcholine receptors. Clinical manifestations are predominantly neurological. Envenomation can cause lethargy, confusion, salivation, cranial nerve palsies, and respiratory paralysis. Symptoms are usually delayed, up to 12 hours from the initial bite. Coagulopathy and tissue necrosis does not happen with coral snake venom [2]. Unfortunately, the Elapidae antivenom is no longer manufactured in the United States and there is a limited supply available.
ED Work Up
As in all patients who present to the emergency department, first ensure that airway, breathing, and circulation are intact. All suspected snake bite injuries warrant a prompt toxicology or poison center consult.
Sometimes, patients will bring in a dead or decapitated snake for identification in the emergency department. DO NOT attempt to handle a snake the patient brought in for identification, even if it is dead. Many snakes have intact reflexes that are preserved even after death or decapitation and you can still be bitten and envenomated by a dead snake!
Examine the injury and look for clear fang marks or puncture wounds. Get a history focused on the timing of the injury, medication allergies, and description of the snake, if known. The borders of erythema should be measured and marked serially.
Laboratory work-up is focused on assessing coagulopathy and hemolysis, especially if the snake is a confirmed pit viper or is unknown. Obtain CBC with platelet count, PT, PTT, INR, fibrinogen, and D-dimer. It is also important to check a baseline set of electrolytes with a basic chem panel, assess the extent of myonecrosis with a CK, and assess for renal damage with a UA.
Manage the wound with copious irrigation and exploration for retained foreign bodies (ie. fangs or teeth). Inquire about the patient’s tetanus status and administer if they are not up to date. Do not attempt to tourniquet or suction venom out of the wound. There is no evidence for routine antibiotic use in snake injuries [6].
Crotaline Bite Management
Consider using CroFab antivenom if the local area of injury and erythema is expanding. If coagulopathy is detected, do not treat with heparin or FFP. Give antivenom first, as unneutralized venom will react with clotting factor replacements [2]. Patients with abnormal coagulation studies within 12 hours after CroFab administration are more likely to develop recurrent coagulopathy. In these patients, repeat coagulation studies should be obtained every 48 hours until resolved. If lab values are worsening, then antivenom retreatment should be reconsidered [7].
Observe the affected limb for compartment syndrome. If clinical suspicion is high for compartment syndrome, consider formally measuring compartment pressures. Elevate the affected limb, and administer extra vials of antivenom. Antivenom administration is preferred over fasciotomy in the treatment of compartment syndrome caused by Crotaline venom [8].
Crofab, the Crotaline antivenom, is typically administered in stepwise fashion and is titrated to clinical resolution of symptoms. Administer 4-6 vials of CroFab antivenom and watch for clinical improvement at the local site of injury. If no improvement seen, administer 4-6 more vials. Repeat until control is achieved, meaning a reversal of symptoms, such as erythema, swelling, pain. Then administer 2 vial doses 6 hours later, then 12 hours, then 18 hours. Envenomation patients should be monitored for at least 8 hours. Keep epinephrine and antihistamines nearby in case of anaphylaxis or allergy to antivenom [2].
Elapidae Bite Management
Because of their potential devastating neurologic effects, coral snake bites should be empirically treated with antivenom and monitored for respiratory deterioration. Provide good supportive care, including intubation and ventilation, if necessary. Avoid opioids for pain management as they may mask symptoms of impending neurologic manifestations. Patients with suspected coral snake envenomations should be monitored for 12 hours after the initial bite [2].
Expert Commentary
Thank you, Dr. Lima for bringing the important and timely topic of snakebites to the table by posting this excellent overview! Current poison center data (2018 National Poison Data System) indicate a total of 4,013 crotalid exposures with the majority being copperheads. While morbidity is worrisome, mortality was fortunately low in our country with only one fatality reportedly from a rattlesnake [1].
Prehospital snakebite management has been an area of deserved scrutiny. Limb immobilization, analgesia, and transport to a medical facility are critical actions. Tourniquets, pressure immobilization bandages, cryotherapy, electrotherapy, and incision/suction are not recommended and are likely harmful. One researcher discovered that venom extraction suction devices “just suck” [2]. Having a cell phone in the field is most important to prevent loss of limb or life!
In other regions of the world, capturing or killing the snake may be optimal in determining which species specific antivenom to administer. For North American crotalids, however, this practice is discouraged and exceedingly dangerous. Both CroFab and Anavip (recently approved and now marketed with the goal of reducing risks of late coagulopathy) are prepared from several species of North American crotalids and can be used to manage any crotalid envenomation. These contemporary antivenoms (Fab fragments) are safer than older polyvalent antivenom that resulted in high rates of anaphylaxis.
Consult your regional poison center (1-800-222-1222) or staff medical toxicologist when managing snakebites! For the number of snakebites that present to the emergency department, poison centers manage severalfold more each year. Making decisions regarding the management of a limb that resembles compartment syndrome (more antivenom vs. surgical consultation), the interpretation of laboratory results, redosing of antivenom to gain initial control of swelling, and the management of nonindigenous (e.g. cobras, gaboon vibers) pet snakebites are nuances your subspecialists would love to collaborate on!
References
1. Gummin DD, Mowry JB, Spyker DA, BrooksDE, Beuhler MC, RiversLJ, Hashem HA, & Ryan ML 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report, Clinical Toxicology, 2019;57:1220-1413.
2. Bush SP. Snakebite Suction Devices Don’t Remove Venom: They Just Suck. Annals of Emergency Medicine, 2004;43:187-188.
Sean Bryant, MD
Assistant Director, Toxicology Fellowship Program, Department of Emergency Medicine, Cook County Health
Associate Professor, Department of Emergency Medicine, Rush Medical College
How To Cite This Post:
[Peer-Reviewed, Web Publication] Lima, R. Cornardy, M. (2020, Oct 26). Management of Snake Bite Injuries. [NUEM Blog. Expert Commentary by Bryant, S]. Retrieved from http://www.nuemblog.com/blog/snake-bites.
Other Posts You May Enjoy
References
Snakebite Injuries Treated in United States Emergency Departments, 2001–2004. O’Neil, Mary Elizabeth et al. Wilderness & Environmental Medicine, Volume 18, Issue 4, 281 - 287
Gold, Barry S., et al. “Bites of Venomous Snakes.” New England Journal of Medicine, vol. 347, no. 5, 1 Aug. 2002, pp. 347–356., doi:10.1056/nejmra013477.
Seifert, Steven A., et al. “AAPCC Database Characterization of Native U.S. Venomous Snake Exposures, 2001–2005.” Clinical Toxicology, vol. 47, no. 4, 2009, pp. 327–335., doi:10.1080/15563650902870277.
“Venomous snakes distribution and species risk categories.” World Health Organization. 2010. http://apps.who.int/bloodproducts/snakeantivenoms/database/
Cardwell, Michael D. “Recognizing Dangerous Snakes in the United States and Canada: A Novel 3-Step Identification Method.” Wilderness & Environmental Medicine, vol. 22, no. 4, 1 Oct. 2011, pp. 304–308., doi:10.1016/j.wem.2011.07.001.
Prophylactic Antibiotics Are Not Needed Following Rattlesnake Bites. August, Jessica A. et al. The American Journal of Medicine, Volume 131, Issue 11, 1367 - 1371
Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Annals of Emergency Medicine, 2001, Vol.37(2), p.196-201., doi: 10.1067/mem.2001.113134
Hall, Edward L. “Role of Surgical Intervention in the Management of Crotaline Snake Envenomation.” Annals of Emergency Medicine, vol. 37, no. 2, Feb. 2001, pp. 175–180., doi:10.1067/mem.2001.113373.
add tags
schedule post
share to twitter u%
format other posts you may enjoy
fix URL and copy to google file
U Tox: Clinical Utility and False Negatives
Written by: Ben Kiesel , MD (NUEM ‘23) Edited by: Jason Chodakowski, MD (NUEM ‘19) Expert Commentary by: Joe Kennedy, MD
Expert Commentary
The urine drug screen is one of the most frequently ordered and more frequently cursed tests in all of medicine. It is one of the few tests that generates more argument than the banal discussions between surgeons and emergency physicians regarding the utility of a white blood cell count. Nevertheless, in skilled hands, this cheap and simple immunoassay can answer a few quick questions. The key is in knowing your Achilles heel:
Opioids and opiates produce similar toxidromes, but there are virtually limitless combinations of street and prescription drugs, lab assays, and confirmatory tests to tell these apart. If the test is negative, who cares! Treat the patient in front of you. Fentanyl and its analogues are perhaps most often missed. Wake the patient up, discuss their substance use, and move on.
Hopefully you noticed that *many* things other than phencyclidine can lead to a positive urine drug screen for PCP. Ever have a conversation with the mother of a 12 year old about their PCP use? Best go into that conversation knowing that many other substances cross-react. Perhaps the kid is actually on lamotrigine for seizures, and that is why they were combative. Or perhaps like most humans, they took a few ibuprofen at some point. Either way—when child protective services is consulted for a positive result, either call the poison center or your local toxicologist for help.
Perhaps the most important point is that this is a screen and absolutely by no means a confirmatory test. When it matters (placement, custody, fired/hired, transplant eligibility, excluding other diagnoses), get an actual level or result. Not sure how to do that? Call your lab! Or call me. Toxicologists love solving these problems and teaching and helping others do the same!
Joe Kennedy, MD
Attending Physician, Emergency Medicine
University of Illinois Hospital
Senior Toxicology Fellow
Toxikon Consortium
How To Cite This Post:
[Peer-Reviewed, Web Publication] Kiesel, B. Chodakowski, J. (2020, Oct 19). U Tox: Clinical Utility and False Negatives. [NUEM Blog. Expert Commentary by Kennedy, J]. Retrieved from http://www.nuemblog.com/blog/utox-clinical-utility-and-false-negatives.
Other Posts You May Enjoy
Mood Stabilizer Toxicities
Written by: Justine Ko, MD (NUEM PGY-4) Edited by: Sarah Dhake, MD (NUEM ‘19) Expert Commentary by: Patrick Lank, MD, MS
Expert Commentary
This is a great summary of the causes, symptoms, work-up, and treatment of two relatively common medications that cause toxicity. In fact, these (along with carbamazepine) are levels I routinely recommend checking in patients with a history of bipolar disorder who come to the emergency department with altered mental status even if they do not report a history of being on these medications. That is because these three medications are very commonly used in the treatment of bipolar disorder and all have quite different treatment courses. In addition to the great summary above, below are some of my usual teaching points about these medications in overdose.
Let's tackle them separately as they are quite different toxicities.
First let's talk about lithium. In almost all medical texts, the tissue distribution of lithium is appropriately identified as being "complex." The easiest way I communicate that with patients, families, and medical learners is that in chronic therapy, lithium forms "stores" of drug in the body and intracellularly. Clinically that is relevant because after performing hemodialysis (HD) for lithium toxicity, you will reliably see an initial drop in lithium concentration followed by elevation approaching pre-dialysis levels if routine HD is performed. Although that could make one feel ambivalent about routinely recommending HD for lithium toxicity, there is suggestion of an alternate advantage of performing HD for lithium toxicity. Vodovar et al published a study in 2016 showing that patients who met their institutional criteria for HD and had HD performed had significantly fewer neurologic side effects from their toxicity than those who met criteria but did not have HD. So even though it did not impact usual measurements that we would expect HD to influence -- mortality and ICU length of stay -- its performance in this study seems to have been clinically beneficial.
The other big thing to discuss with lithium toxicity is that there are many known medication interactions with lithium. In short, any medications that impair renal function should not be used in someone on chronic lithium therapy. The main list of those medications includes NSAIDs, ACE inhibitors, ARBs, and thiazide diuretics.
Most of the unique aspects of valproate toxicity focus on its diagnosis and treatment. In the setting of acute intentional overdose of valproate, one of the most important things for emergency physicians to be aware of is that there can be a delay of peak valproic acid level. There are cases of patients presenting to an emergency department with stated valproate ingestion, initial negative level, then repeat level hours later being in the toxic range. So I recommend serial valproate levels until both down-trending and non-toxic. For treatment, there is a great summary of recommendations by the Extracorporeal Treatments in Poisoning Workgroup (EXTRIP) published online (https://www.extrip-workgroup.org/valproic-acid). In short, consult nephrology for dialysis if the patient is super sick.
As always, I recommend you consult your regional poison center when you are worried your patient is experiencing medication toxicity. But I hope this infographic and some of my comments helps you understand their recommendations.
References
Vodovar V, et al. Lithium poisoning in the intensive care unit: predictive factors of severity and indications for extracorporeal toxin removal to improve outcome. Clin Tox (Phila) 2016 Sep; 54(8): 615-23.
Lank P and Bryant S. "Valproic Acid" In: Wolfson A, Hendey G, Ling L, Rosen C, Schaider J, Cloutier R (eds.): Harwood-Nuss’ Clinical Practice of Emergency Medicine, 6th edition. Lippincott Williams & Wilkins 2014.
Patrick Lank, MD, MS
Assistant Professor of Emergency Medicine
Medical Toxicologist
Department of Emergency Medicine
How To Cite This Post
[Peer-Reviewed, Web Publication] Ko J, Dhake S. (2020, July 13). Mood Stabilizer Toxicities [NUEM Blog. Expert Commentary by Lank P. Retrieved from http://www.nuemblog.com/blog/mood-stabilizer-tox