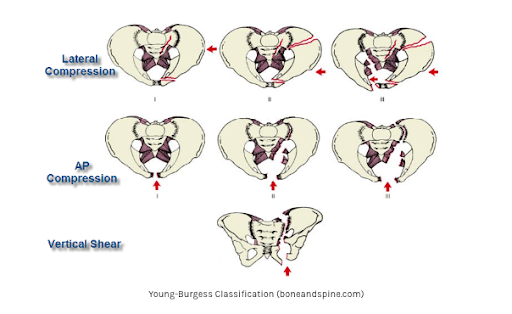
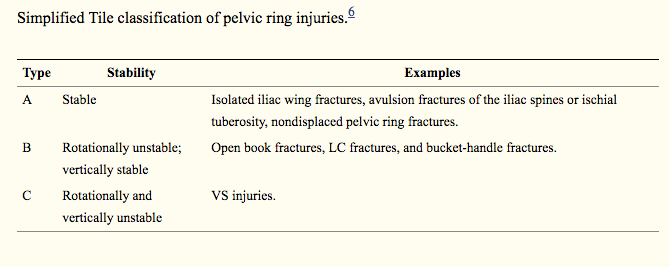
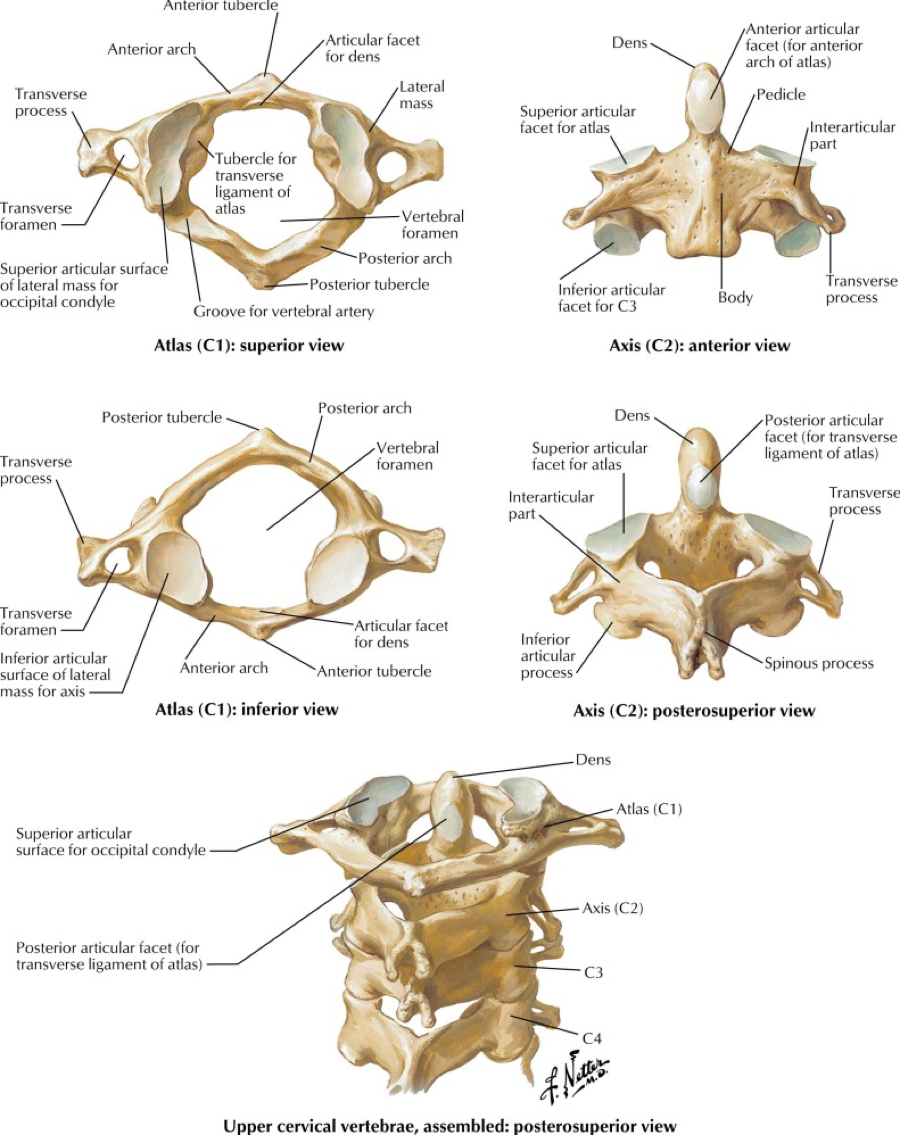
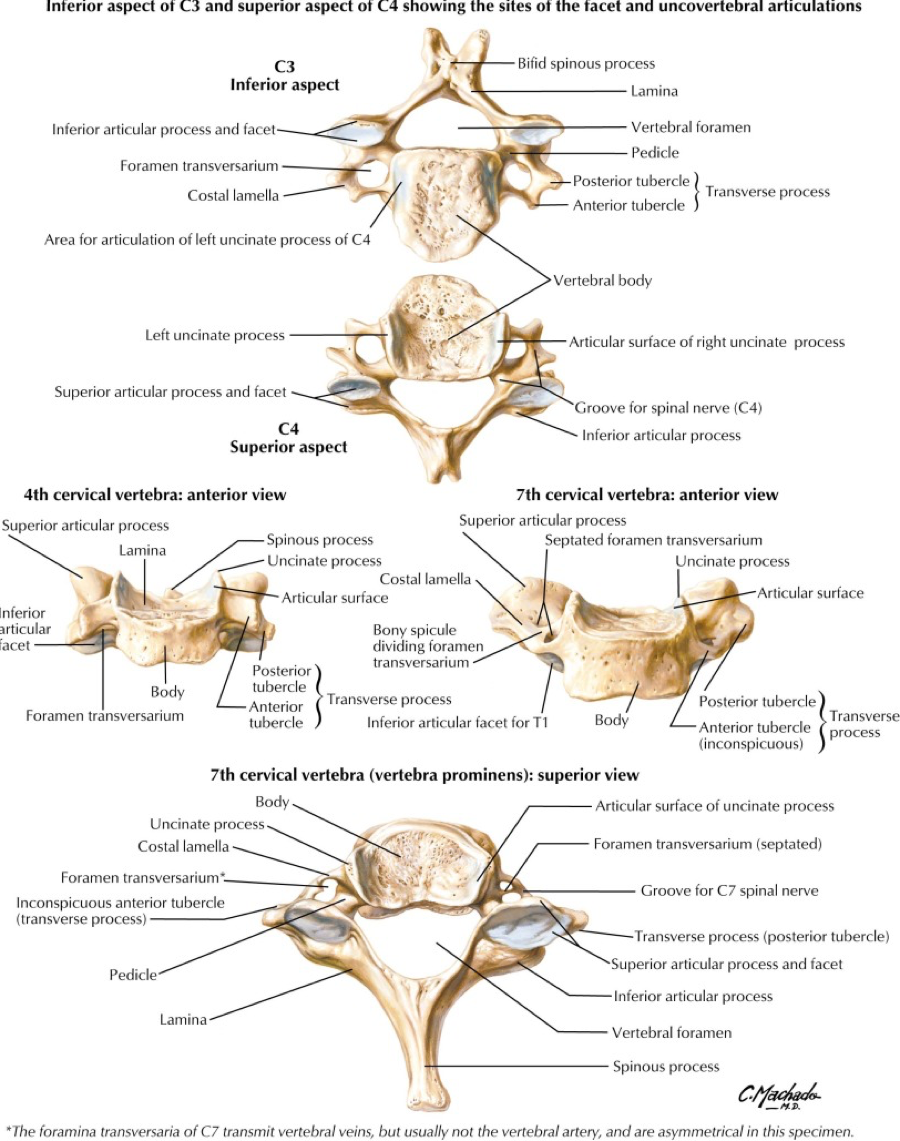
An overview of Massive Transfusion Protocol
TXA in the Trauma Bay
Written by: Jilan Shimberg, MD (NUEM ‘26) Edited by: Rafael Lima, MD (NUEM ‘23)
Expert Commentary by: Matthew R Levine, MD
Expert Commentary
Unlike many of the treatments and interventions we use in the Emergency Department and the trauma bay, tranexamic acid (TXA) has rather robust studies to guide usage. Like many interventions, however, even when there are studies with large numbers of patients and positive results, there are still barriers towards implementation. TXA is no different.
Working at a Level 1 Trauma Center and frequently interacting closely with the trauma surgeons through the Trauma Quality Management Committee, I often follow their lead when it comes to promising trauma innovations through the years such as TXA, REBOA, permissive hypotension, and so on. What I have observed is that our trauma surgeons tend to believe that there is benefit to properly timed TXA in the right trauma patients and that we do not use it enough. Yet use of TXA in trauma at our hospital has not been protocoled.
Why not? Some possibilities:
Someone usually (but not always) thinks to give it to patients who would benefit despite there not being a protocol (thanks ED pharmacists!).
The patients who need it most also need something else even more – source control of hemorrhage. Anything that slows or distracts from that may be counterproductive. It may not seem like a simple TXA infusion would delay anything. But keep in mind the multiple lines sick trauma patients may need and the often already chaotic nature of “the bay” getting the sickest patients the tubes, meds, lines, products, studies, and, ultimately, proper disposition during their “golden hour”. The nurses have many tasks, to say the least. But maybe this is an argument for why use of TXA should just be protocolized.
I bounced this off of our trauma section head to make sure I was not misrepresenting their thoughts. As a result, we are looking into protocolling its use. Thanks NUEM Blog!
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Shimberg, J. Lima, R. (2024, Mar 11). TXA in the Trauma Bay. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/txa-trauma
Other Posts You May Enjoy
Debriefing in the ED
Written by: Diana Halloran (NUEM ‘24) & Andrew Long (NUEM ‘25) Edited by: Nick Wleklinski (NUEM ‘22)
Expert Commentary by: John Bailitz, MD
Introduction
The Emergency Department is a challenging work environment for a variety of reasons. It is not surprising that unpredictable work hours mixed with frequent interactions with patients undergoing physical and emotional trauma can cause spillover into the personal lives of ED staff. However, in the recent years there has been an increasing body of literature that highlights the challenges that we face as ED personnel.
The American Heart Association even recommends a “hot” debrief for every cardiac arrest attended by a healthcare professional. We know debriefing has concrete and tangible advantages. Previous studies have shown that debriefing immediately after an event can improve individual and team performance by 20-25%, issues can be identified for interventions, and mental health and emotional trauma can be addressed. It is well-documented that ED physicians have historically high rates of burnout compared to other specialties as well as a high incidence of post-traumatic stress disorder (PTSD) compared to the general population. PTSD is 2 times more prevalent in physicians (14.8%), with EM resident physicians falling in the range from 11.9%-21.5%.
As a field, we are now starting to design and implement interventions to preserve our own mental wellbeing. In fact, debriefing has been shown to be one method for managing PTSD. One article described an initiative that implemented an immediate (also known as “hot”) debrief protocol immediately following all cardiac arrests during which 90% of participants felt they benefited psychologically, and 100% felt it improved their clinical practice. In this post, we will give a brief overview of a landmark study in the ED debrief literature, which advocates for a “hot” debrief model in resuscitation cases using the STOP5 model.
Study Analysis
This study published in 2019 designed, tested, and developed the STOP5 model to facilitate safer patient care, team development, and quality improvement within the Emergency Department. The STOP5 model, the first widespread debriefing tool, was designed as a “hot debrief”: “an interactive and structured team dialogue that takes place either immediately or very shortly after a clinical case”. Any team member of the resuscitation may lead the hot debrief.
Figure 1: The STOP5 Model Debriefing Framework (proposed by CA Walker et al 2020)
After an initial check-in the team moves to a group discussion and follows the STOP framework above: Summarize the case, Things that went well, Opportunities to improve, and Points to action and responsibilities. Inclusion criteria for these hot debriefing cases were major traumas, deaths in resuscitation, and any cases upon request by any staff member. No potential resuscitation cases were formally excluded.
After 18 months the ER staff was re-surveyed to ascertain STOP5 rating scores, the number of staff involved in the debriefs, any possible benefits or barriers to team performance, and if staff believed there should be more or less hot debriefing in the ER. In this 18-month review all STOP5 debriefs were rated “good” to “excellent”, suggesting the debriefing was highly valued. 98% of respondents believed that there should continue to be more hot debriefs in the emergency department. In a 12-month review there were 10 process and equipment changes (“hard outcomes”) as a direct result of the STOP5 hot debriefs and 14 additional opportunities for improvement. The hot debrief allowed for concrete actions to be taken about these issues and for a dedicated plan of action for correction. These hard outcomes identified issues such as those listed below which allowed for concrete solutions for all the identified problems.
Shortage of resuscitation room equipment
Drug stocking issues
Drug preparation/infusion regime for vital but rarely used medications difficult for staff to find
Faulty equipment (doors, machines)
Reported barriers to enacting hot debriefs include time constraints, workload, low staff confidence in leading the debrief, or absence of team members (consultants who might have left the department, change of shift). For these reasons hot debriefing is still not standard practice. However, a hot debrief such as STOP5, with a concrete checklist, is an inexpensive and quick way to enhance team performance, improve patient care, and assist with emotional trauma and mental check-ins for the team. Debriefing is a valuable and important aspect of our medical career. We hope to bring more of a focus to debriefing within our institution by beginning to enact the STOP5 based hot debriefing after clinical events.
Expert Commentary
Whether working in a community or academic Emergency Department, recurrent extraordinary cases threaten the well-being of the clinical team during that shift and after. Although resources often exist for individual employee assistance after a difficult shift has ended, few interventions have been described to help the team regroup and recover during that particular shift. Furthermore, department level morbidity and mortality conferences or hospital level quality assurance reviews focus more on the technical case details and less on team wellness.
With the primary purpose of quickly restoring team performance and wellness, hot debriefs at NUEM provide the opportunity for our ED teams to have a structured yet brief meeting immediately after an extraordinary case. Using STOP5, team leaders have a step-by-step plan to quickly yet effectively help every clinician on the team properly mentally frame the case, share gratitude, and then identify and assign opportunities for immediate improvement. Building on life support courses and residency training, specific education on the STOP5 framework quickly prepares senior clinicians to lead hot debriefs. Utilizing change management principles to identify and address logistical barriers helps to create a culture that supports immediate debriefing. Successful strategies in our NUEM ED include protocols to pause new inflow and cover existing patient demands, designating meeting spaces, adding positive program reminders to clinical areas and recurring meetings, and tracking and celebrating program success.
References
1. Get With The Guidelines - Resuscitation Clinical Tools. (2021, August 16). Www.Heart.Org. https://www.heart.org/en/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-resuscitation/get-with-the-guidelines-resuscitation-clinical-tools
2. Gilmartin, S., Martin, L., Kenny, S., Callanan, I., & Salter, N. (2020). Promoting hot debriefing in an emergency department. BMJ Open Quality, 9(3), e000913. https://doi.org/10.1136/bmjoq-2020-000913
3. Tannenbaum, S. I., & Cerasoli, C. P. (2012). Do Team and Individual Debriefs Enhance Performance? A Meta-Analysis. Human Factors: The Journal of the Human Factors and Ergonomics Society, 55(1), 231–245. https://doi.org/10.1177/0018720812448394
4. Vanyo, L., Sorge, R., Chen, A., & Lakoff, D. (2017). Posttraumatic Stress Disorder in Emergency Medicine Residents. Annals of Emergency Medicine, 70(6), 898–903. https://doi.org/10.1016/j.annemergmed.2017.07.010
5. Walker, C. A., McGregor, L., Taylor, C., & Robinson, S. (2020). STOP5: a hot debrief model for resuscitation cases in the emergency department. Clinical and Experimental Emergency Medicine, 7(4), 259–266. https://doi.org/10.15441/ceem.19.086
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Halloran, D. Long, A. Wleklinski, N. (2022, Sept 12). Debriefing in the ED. [NUEM Blog. Expert Commentary by Bailitz, J]. Retrieved from http://www.nuemblog.com/blog/debriefing-ED
Other Posts You May Enjoy
Proper Preparation for Mass Casualty Incidents
Written by: August Grace, MD (NUEM ‘24) Edited by: Andrew Rogers, MD, MBA (NUEM ‘22)
Expert Commentary by: Andra Farcas, MD (NUEM ‘21)
Introduction
In the setting of trauma, most hospitals are adept at treating and managing patients with a variety of injuries. However, the ability of a hospital to handle a mass casualty incident (MCI) requires a completely different approach and, most importantly, adequate triage and pre-planning. An MCI is defined as “an event that overwhelms the local healthcare system, where the number of casualties vastly exceeds the local resources and capabilities in a short period of time [7].” MCI events can include anything from hurricanes, earthquakes, and other natural disasters to terrorism or other man-made situations that include the use of explosive or biological weapons, mass shootings, or dysfunction in modes of transportation (car, plane, train crash) [3]. Although this is just a short list of the possibilities, each hospital must prioritize its response preparedness to match the likelihood of events that it could receive. For example, as a major urban center, Chicago is more likely to encounter events such as mass shootings, biological terrorism, or explosive injuries. Mobile, Alabama, on the other hand, must have adequate preparation for hurricanes and floods [4]. This post will discuss a brief overview of hospital planning and operational setup with key elements of a disaster response from events that cause high numbers of blunt trauma, penetrating trauma, burns or crush injuries that may be seen following explosive events, mass shootings, or large scale motor vehicle collisions, to name a few.
Casualty Planning & Staffing Considerations
Arguably the most important step in an MCI is the planning that occurs before a single patient is even seen. In most events, hospitals have communication with EMS personnel that are on scene allowing them to have some sort of estimation of the scale of the event and type of disaster encountered. If the mechanism and scale are appropriate, a properly planned disaster response should be initiated and set in motion a sequence of coordinated events.
In creating a disaster response plan, the first step is the designation of the Disaster Medical Officer (DMO). This person should be the most senior ED attending physician and he/she oversees available hospital medical personnel and resources [1]. This person will have no role in patient care and instead will be in charge of all ED operations, delegating tasks, and problem solving issues that arise in the future.
The first task of the DMO is to get help and get it now. Approximately half of all casualties will arrive at the hospital within a one hour window, with 50-80% arriving within 90 minutes. Time begins after the first patient arrives at the hospital [8]. Therefore, getting the appropriate staff to the hospital as quickly as possible is vital to saving lives. How much staff is needed? This can be gauged by the type of disaster encountered and with assistance from EMS personnel at the scene. A five-car motor vehicle collision (MVC) will not require as much additional staff as a collapsed high rise building. The DMO will delegate the task of calling available staff to maximize the number of staff present in the ED and hospital. Contacting surgeons, scrub techs, anesthesiologists, and nurses to get as many ORs operational is invaluable to saving lives.
Continuous staffing adjustments can be monitored and made by using the casualty predictor tool (Figure 2). When in doubt, it is better to have more staff available than needed. To reiterate, the most important part of any disaster situation is to GET HELP.
Triage/ED Setup
Once the process is underway for increasing the level of resources available, the next step is hospital setup and triage. The most important part of this step is creating enough space to allow for the massive influx of patients and maintaining proper flow throughout the ED. It is well known that most hospitals in large population centers already operate at or near full capacity [4]. This makes it even more challenging when presented with an acute influx of patients in a short period of time. There is not much that can be done in the acute setting about patients that are already admitted; however, the ED can be restructured to account for the increased surge. Patients currently in the ED with a condition deemed to be stable (will likely not require an acute intervention in the next 24 hours) can be moved to a different area (green triage area, discussed below). The patients who have a more acute condition can be triaged and recategorized using the same criteria as the incoming casualties.
One current method of triaging patients is the tagging method. In this system, patients are tagged with a red, yellow, or green identification that categorizes patients based on acuity. Other things listed on tags can be a patient’s name, bar code, MRN or other tracking criteria. These patients are then able to be treated based on the level of care needed. In theory, this is a good way for patients to be tracked and accounted for. However, some experts believe that when there is a large volume of patients, this can slow the triage process and extend the amount of time it takes the patient to receive care that may be lifesaving. Another limitation is that it does not allow for a dynamic system, it provides a false sense of security, and can cause confusion. For example, a patient may have a green tag when initially triaged but could decompensate to a yellow or red tag [6]. Thus, there should be an appropriate system for re-evaluation if resources allow.
One system that could be used instead is a tag zone. In this system, the ED could be set up into different zones that would correlate with the tag color and acuity of the condition. Who should triage? The second most senior ED attending physician. The zone system could be set up as follows:
Red zone: Patients that need immediate medical or surgical attention. This includes patients presenting with an acute airway, circulatory or neurologic problem, multi-system involvement, or penetrating injuries to the head, neck, or chest. These patients are likely to need the vast majority of resources and staff.
Orange zone: Not originally categorized in the tag system. These patients are expected to decompensate within the hour but did not need immediate resuscitation [2].
Yellow zone: Patients that are relatively stable that will likely not decompensate within the hour. Extremity injuries or conditions that have time to be worked up.
Green zone: Patients with minor injuries that are unlikely to decompensate. “Walking wounded.” Will not require vast amounts of resources or staffing to be cared for.
The different zones allow for a dynamic system. Patients in each zone will be cared for by a team of physicians and nurses with the majority of staffing located in Red and Orange zones. Patients can be moved “up” a zone (from yellow to orange) if their condition deteriorates or could be moved “down” a zone (from yellow to green) if they are able to be stabilized [1, 2, 3]. The “black tag” patients were not categorized into a zone as they were patients who were either already dead, or not likely to survive given the current staffing and resources available. Figure 3 shows a brief triaging algorithm (without the orange designation). This is one possible system to triage patients and get them to an appropriate level of care rapidly. The hospital system is now ready for the rapid influx of critical patients.
Implementation
All patients should enter through a single triage area. Multiple points of entry can cause confusion and overwhelm each area by not knowing the number of patients entering from each point [4]. As the number of patients in each zone starts to fill up, adequate communication about the space and number of resources available should be communicated to the DMO and charge nurse. Once patients are able to be stabilized in each zone, the goal is to get them to the OR for immediate surgery if needed, or to move them down a zone (yellow to green) in order to make space for additional critically injured patients.
Who are the first patients to arrive? The “dual wave phenomenon” explains how patients usually present to the hospital following MCIs. The first wave of casualties are described as the “walking wounded” or those who are able to self-ambulate and usually only require minor care. These patients begin to arrive within 15-30 minutes of the incident depending on the distance from the scene to the hospital. It is important that these patients do not take up too many hospital resources or staff as they are likely well enough to survive with minimal therapeutic interventions. These patients can easily overwhelm the system and prevent proper care to more critical patients. The second wave includes the patients that arrive via EMS or other assistance from bystanders as they are not well enough to transport themselves. These are the patients who will require a vast majority of hospital staffing, resources, and time in order to prevent deaths [3, 4].
Summary
The first step to preparing for an MCI is having a plan in place.
GET HELP. If you only have time to do one thing it should be this. It does not matter how many resources you have or how much space is available if you do not have enough staff to use them.
Have a triage plan. Create zones of various acuity with the majority of staff occupying the higher acuity areas. Patients can always be moved to a higher zone if they need more care or a lower zone if they have been stabilized.
Get patients to the proper provider. If the patient needs surgery, get them to the OR. This also creates space for new patients to be seen.
Have one single area of entry. This allows the system to maintain consistency and flow.
References
Emergency Safety Officer Management Plan For Mass Casualty. Kings County Hospital Center, www.downstate.edu/emergency_medicine/pdf/KCHCSection03.pdf.
Menes, Kevin. “How One Las Vegas ED Saved Hundreds of Lives After the Worst Mass Shooting in U.S. History: Emergency Physicians Monthly.” EPM, 5 Apr. 2020, epmonthly.com/article/not-heroes-wear-capes-one-las-vegas-ed-saved-hundreds-lives-worst-mass-shooting-u-s-history/.
Hospital Medical Surge Planning for Mass Casualty Incidents. Florida Department of Health, www.urmc.rochester.edu/MediaLibraries/URMCMedia/flrtc/documents/WNY-Hospital-Medical-Surge-Planning-For-Mass-Casualty-Incidents.pdf.
Institute of Medicine. 2007. Hospital-Based Emergency Care: At the Breaking Point. Washington, DC: The National Academies Press. https://doi.org/10.17226/11621
“SALT Mass Casualty Triage Algorithm - CHEMM.” U.S. National Library of Medicine, National Institutes of Health, chemm.nlm.nih.gov/salttriage.htm.
“Report: Mass Casualty Trauma Triage Paradigms and Pitfalls .” Journal of Emergency Medical Services , Office of the United States Assistant Secretary for Preparedness and Disaster Response.
DeNolf, Renee L. “EMS Mass Casualty Management.” StatPearls [Internet]., U.S. National Library of Medicine, 15 Oct. 2020, www.ncbi.nlm.nih.gov/books/NBK482373/.
“Mass Casualty Predictor .” Homeland Security Digital Library , Centers for Disease Control and Prevention .
Expert Commentary
This is a great review of MCI management in the Emergency Department by Drs. Grace and Rogers. Although the past few years of the COVID-19 pandemic have felt like we’ve been working in a perpetual MCI, these are important principles to review on a regular basis, as they are not something we necessarily practice every day in the emergency department.
The authors do a good job of emphasizing the importance of preparing for an MCI ahead of time. Another important aspect of preparation is decontamination. An ED disaster response plan should incorporate how to effectively put patients (both walk-ins and EMS arrivals) through decontamination if the disaster at hand requires it. The authors emphasize the importance of having patients enter through a single triage area, and the decontamination station should be similarly set up nearby allowing for one-directional flow of patients through the decontamination process. This is not only vital to patient treatment but also to ensuring staff safety. Additionally, it is necessary to ensure that the ED has sufficient and adequate level Personal Protective Equipment and that the appropriate staff are trained on donning/doffing procedures.
In addition to gathering the staffing resources, there should also be an emphasis on gathering disaster-specific supplies: alerting the blood bank if it is a traumatic MCI, amassing antidotes if it is toxicological in nature, compiling medical equipment (such as ventilators) as applicable, etc. Additionally, alerting other EDs in the system as to the impending influx of patients as well as reaching out to disaster-specific specialty centers (ie, hyperbarics facility for a structure fire for carbon monoxide treatment) can also help take pressure off and allocate more resources.
Finally, the importance of a hotwash or after-action review cannot be emphasized enough. This is a process by which participants can have an open and honest professional discussion about what went well and what can be improved in the future. It centers around four main questions (What was supposed to happen? What did happen? What caused the difference? What can we learn from this?) and is vital for building an ED’s capacity for conducting an adequate emergency response to an MCI.
References
Blackwell, T.H., DeAtley, C., Yee, A. (2021). Medical support for hazardous materials response. Cone, D.C. (ed). Emergency Medical Services Clinical Practice and Systems Oversight; Volume 2: Medical Oversight of EMS. (3rd edition, p339-351). UK: John Wiley and Sons, Ltd.
Greenberg, T., Adini, B., Eden, F., Chen, T., Ankri, T., Aharonson-Daniel, L. An after-action review tool for EDs: learning from mass casualty incidents. Am J Emerg Med. May 2013;31(5):798-802. Doi 10.1016/j.ajem.2013.01.025. Epub 2013 Mar 6. PMID: 23481154.
Metz, T. How to Facilitate an After-Action Review (AAR or Hot Wash): Agenda and Tips. MG Rush Facilitation Training & Meeting Design. https://mgrush.com/blog/after-action-review/.
Salem-Schatz, S., Ordin, D., Mittman, B. Guide to the after action review. Center for Evidence-Based Management. Oct 2010. https://www.cebma.org/wp-content/uploads/Guide-to-the-after_action_review.pdf.
Andra Farcas, MD
Emergency Medicine & EMS Physician
CU Department of Emergency Medicine
University of Colorado School of Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Grace, A. Rogers, A. (2021, Apr 26). Proper Preparation for Mass Casualty Incidents. [NUEM Blog. Expert Commentary by Farcas, A]. Retrieved from http://www.nuemblog.com/blog/mass-casualty-incident-preparation
Other Posts You May Enjoy
C-Spine Intubation
Written by: Daniel Levine, MD (NUEM ‘24) Edited by: Zach Schmitz (NUEM ‘21)
Expert Commentary by: Matt Levine, MD
The “Evidence” Behind Manual In-Line Stabilization During Intubation of Trauma Patients
Background
Even in the absence of frank head and neck trauma that may cause bleeding or distortions in usual anatomy, trauma patients present challenging airways because of cervical spine precautions. Standard-of-care technique according to EAST (Eastern Association for the Surgery of Trauma), West (Western Trauma Association), and ATLS (Advanced Trauma Life Support) guidelines for intubating acute trauma patients with known or potential cervical spine injury involves manual in-line stabilization (MILS). (1,2) This is a two-person technique whereby one provider performs laryngoscopy while another holds the patient’s neck in place. The two most common techniques for this procedure are depicted below, one in which the stabilizer crouches down at the head of the bed (A), and the other where the stabilizer approaches from the side of the bed (B). (3)
(photo from Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition) (3)
Evidence
Like many practices in medicine, MILS has never been studied in randomized controlled trials, and the practice stems more from weak data and expert opinion. (4) The practice of spinal stabilization began during the 1970s after a retrospective review published in 1979 of 300 patients with acute cervical injuries who presented to Johns Hopkins hospital between 1950 and 1972. Although the main focus was on the effects of laminectomy and steroids, the review also found that 11 of the 300 patients developed neurologic deficits after reaching the hospital. Of the 11 patients, 7 developed these deficits “after neck immobilization was not provided”, with no clear comment as to whether immobilization was not provided during intubation or during some other process of the patient’s care. (5) These observations led to concerns that mobilization of the neck during intubation may worsen spinal cord injury, so manual in-line stabilization became standard of care in the 1980s.
Existing data for spinal stabilization comes from trials of cadaveric models, case series, and uninjured patients. Data from cadavers with post-mortem surgically created cervical spine injuries have shown mixed results on the effects of the amount of measured movement at the injured site with versus without MILS. For example, a 1993 study by Donaldson et al. found higher degrees of subluxation and angulation at C5-C6 during orotracheal intubation without MILS compared to with stabilization in five cadaveric specimens with injuries created in that area. (6) On the other hand, a 2001 Lennarson et al. study on cadavers found MILS significantly increased subluxation in C4-C5 during the same movements. (7) While it is somewhat counterintuitive that performing MILS might be associated with increased cervical motion, this may be explained by the laryngoscopist’s need to apply greater force with the laryngoscope in order to obtain an adequate view. This is what Santoni et al. (2009) found in a matched control study of 9 patients undergoing elective surgery. The patients in this study underwent two sequential laryngoscopies and oral intubations with a Macintosh 3 blade. Pressure transducers attached to the end of the blades detected higher maximum pressures at best glottic view with MILS compared to without. (8)
What is more clear in the literature on MILS than its effect on cervical motion is that it impairs glottic visualization and subsequent first pass intubation success. In the aforementioned Donaldson study on cadavers, MILS was shown to have a negative impact on Cormack-Lehane (CL) grade. (6) Similarly, in the aforementioned Santoni et al. study of 9 patients who underwent two sequential intubations with and without MILS, glottic visualization was worse in 6 patients with MILS, and intubation failure occurred in 2 of these 6 patients compared to no intubation failures among these patients when the intubation was performed without MILS. Thiboutot et al. (2008) performed a randomized controlled trial that further demonstrated this effect. In their study, 200 elective surgical patients were randomized to receive MILS or no MILS, and the primary endpoint was rate of failed intubation at 30 seconds with a Mac 3 blade. The rate of failed intubation was half in the MILS group (50%, 47/94), significantly higher compared to the control group (5.7%, 6/105). When they released manual in-line stabilization, they were able to intubate all patients. Secondary outcomes of rate of CL grade 3-4 as well as mean latency to successful intubation were also both significantly higher in the MILS group. (9) Additionally, these data were from patients undergoing elective surgery being intubated in the controlled OR setting by anesthesiologists. It is likely that the rate of failed intubation would be even higher in the chaotic emergency department environment with an acutely injured trauma patient. While 30 seconds is a somewhat arbitrary cutoff for a failed intubation, and it is quite possible many of the patients in the MILS group who “failed” may have been successfully intubated if a longer cut-off time were chosen, hypoxia caused by failed or delayed intubation is associated with poor outcome in central nervous system injury. (10)
Conclusion
In an ideal world, a large-scale randomized controlled trial of trauma patients studying the effects of MILS on mortality and important functional neurologic outcomes would help elucidate the utility of this commonly accepted practice. However realistically, completing such a study has significant obstacles. Cervical spine injuries are relatively rare (4% of trauma injured patients)4 and only a small fraction of those cases involve unstable injuries with potentially salvageable cord function. Thus, a study with sufficient power to detect any meaningful difference in outcomes would take many thousands of patients, many trauma centers, and many years to complete. Perhaps an even larger hurdle is the ethical and medicolegal hurdle of randomizing patients to not getting MILS and possibly putting them at risk of quadriplegia. (4) So what’s a clinician to do when faced with the common scenario of having to intubate a trauma patient? I personally like the approach that Dr. Reuben Strayer discusses in his video “Advanced Airway Management for the Emergency Physician” (link below). (11) To summarize his strategy:
*The exception: in the rare situation where the patient has a highly suspected (e.g. obvious bony deformity, focal neurologic deficit) or known cervical spine injury, Dr. Strayer recommends lowering the threshold to perform a cricothyroidotomy. Additionally, he recommends considering an awake intubation approach in these patients.
Another consideration is intubating using a hyper-angulated video GlideScope, which has been shown to have improved CL views and high rates of intubation success in c-spine immobilized patients. (12) That said, occasionally equipment availability or a bloody airway may preclude the use of video laryngoscopy in the trauma setting.
References
Mayglothling J, Duane TM, Gibbs M, McCunn M, Legome E, Eastman AL, Whelan J, Shah KH; Eastern Association for the Surgery of Trauma. Emergency tracheal intubation immediately following traumatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012 Nov;73(5 Suppl 4).
Brown CVR, Inaba K, Shatz DV, Moore EE, Ciesla D, Sava JA, Alam HB, Brasel K, Vercruysse G, Sperry JL, Rizzo AG, Martin M. Western Trauma Association critical decisions in trauma: airway management in adult trauma patients. Trauma Surg Acute Care Open. 2020 Oct 9;5(1)
Leonard, J et al. "Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition." Chapter 24: Cervical Spine Injury. https://doctorlib.info/pediatric/schafermeyers-pediatric-emergency-medicine/24.html, accessed 5/7/21.
Manoach S, Paladino L. Manual in-line stabilization for acute airway management of suspected cervical spine injury: historical review and current questions. Ann Emerg Med. 2007 Sep;50(3):236-45.
Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119- 1142.
Donaldson WF 3rd, Towers JD, Doctor A, et al. A methodology to evaluate motion of the unstable spine during intubation techniques. Spine. 1993;18:2020-2023
Lennarson PJ, Smith DW, Sawin PD, Todd MM, Sato Y, Traynelis VC. Cervical spinal motion during intubation: efficacy of stabilization maneuvers in the setting of complete segmental instability. J Neurosurg. 2001 Apr;94(2 Suppl):265-70.
Santoni BG, Hindman BJ, Puttlitz CM, Weeks JB, Johnson N, Maktabi MA, Todd MM. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009 Jan;110(1):24-31.
Thiboutot, F et al. Effect of manual in-line stabilization of the C-spine on the rate of difficult orotracheal intubation by direct laryngoscopy; a randomized controlled trial. Can J Anaesth. 2009 Jun;56(6):412-8.
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993 Feb;34(2):216-22.
“Advanced Airway Management for the Emergency Physician”, uploaded by Reuben Strayer, https://vimeo.com/12440392
Bathory I, Frascarolo P, Kern C, Schoettker P. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilisation by a semi-rigid collar. Anaesthesia. 2009 Dec;64(12):1337-41.
Expert Commentary
So once again a review of a significant body of literature leaves a clinical question unanswered, leaving the practitioner to either follow dogma or make one’s own conclusions. Like most of our medical decision making, this is a risk/benefit analysis. So let’s go through the process.
Some background context to keep in mind:
Most cervical spine injury occurs from the initial traumatic event (primary neurologic injury). Secondary neurologic injury is a cascade of events at the cellular level that worsen primary injury and is exacerbated by hypoxia and hypercarbia, which are frequent events in difficult/prolonged intubations. These must be minimized when the brain or c spine are injured!
The movements of the cervical spine that occur during ED care pale in magnitude to the cervical spine motion that caused the primary injury to occur. These likely contribute less to neurologic outcome than secondary neurologic injury from other events during ED care like hypotension, hypoxia, and hypocarbia.
It’s too difficult to intubate with a collar on. It must be carefully and temporarily removed. As Dr. Levine taught us, MILS impairs glottic visualization and first pass intubation success. Dr. Levine also taught us that we don’t know whether the injured cervical spine actually moves less or more with MILS during intubation attempts.
The synthesis:
These factors all lead me to agree with Dr. Strayer’s approach. It is reasonable to minimize cervical spine motion as much as possible, but not at the expense of adequate glottic visualization. Maybe MILS helps minimize motion during intubation. But abandon MILS when glottic visualization is suboptimal because MILS can be contributing to this, leading to hypoxia, hypercarbia, and secondary neurologic injury. Practice MILS only until it is possibly prolonging airway success, because now it is more likely to be harming than helping.
Even more future questions remain. Much of the prior literature is based on use of traditional orotracheal intubation techniques. How much of that knowledge applies to the now widespread use of fiberoptic video intubations (i.e. Glidescope), which may have better first pass success rates and less neck motion? Do we even need to perform MILS for these intubations? Or can we reliably rapidly intubate with MILS and the Glidecope – so we can have our cake and eat it too?
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Levine, D. Schmitz, Z. (2021, Oct 18). C-Spine. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/cervical-spine-intubation
Other Posts You May Enjoy
Managing Minor Thermal Burns in the ED
Written by: Mitch Blenden, MD (NUEM ‘24) Edited by: Vytas Karalius, MD, MPH, MA (NUEM ‘22) Expert Commentary by: Matt Levine, MD
Expert Commentary
Dr. Blenden and Dr. Karalius provided an excellent handy, high-yield, quick reference of thermal burn considerations in the ED. There are some nuances of thermal burn care that I’d like to provide further commentary:
A pitfall is underestimating the severity of the burn when the patient presents within a few hours of the event. Burn appearance evolves over 24-48 hours. What initially appears as erythematous skin can be covered in bullae the next day. Consider a repeat examination in 24-48 hours, or at least discuss with the patient the possibility that this may occur and what to do if it does. Otherwise, if you initially diagnosed the patient with superficial burns and provided only instructions for superficial burns, which require little treatment or follow-up, the patient can be set up for a worse outcome when these burns subsequently declare themselves to be partial thickness.
For years, most non-facial burns were sent home with instructions to use silver sulfadiazine (AKA Silvadene) cream. This would require teaching of how to apply and remove it. The cream needs to be removed daily before applying a new coat (I always sent the patient home with tongue blades to scrape it off). The benefits of this are that it debrides some nonviable tissue when the cream is removed and provides a moist antimicrobial barrier. The down sides are that removal can be painful and some patients have difficulty performing this procedure, which requires teaching. Silver sulfadiazine can also cause skin staining. There is scant evidence recommending one topical antimicrobial over another. For these reasons, practice (including mine) has evolved in many places to simply prescribe whatever antibiotic ointment is on hand for ease of use and less painful and technically challenging application.
Another controversy is whether to debride blisters and bullae or leave them intact. This is another area without definitive evidence and practice is often guided by gestalt, local custom, or prior teachings. On one hand, intact bullae can be thought of as “sterile” coverings and may be less painful than dermal layers exposed to air and friction. On the other hand, when bullae rupture, the patient is left with dead skin which can be a nidus for infection. My practice has been to leave small blisters intact and debride large bullae if it seems like they will soon rupture and leave the patient with hanging skin fragments. If the patient has reliable follow up burn care then I may choose a less aggressive approach in debriding. Other clinicians are likely to give alternate approaches so ask your attendings what they do in these scenarios so you can develop a practice pattern that makes sense to you.
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Blenden, M. Karalius, V. (2021, Oct 18). Managing Minor Thermal Burns in the ED. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/managing-minor-thermal-burns
Other Posts You May Enjoy
SonoPro Tips and Tricks for Aortic Aneurysm and Dissection
Written by: John Li, MD (NUEM ‘24) Edited by: Andra Farcas, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Aortic ultrasound is a staple in emergency point of care ultrasound. It has incredible sensitivity (97.5-100%) and specificity (94.1-100%) in detecting abdominal aortic aneurysms and can provide a diagnosis for critically ill patients in seconds. [1-4] However, it can often be a technically difficult study for beginner sonographers due to shadowing bowel gas and patient body habitus. Follow along in this installment of our Sono Pro Tips and Tricks Series to become an expert in finding aortas!
Beyond the classic elderly male smoker with abdominal, flank, or back pain, what are other scenarios where you would use aortic ultrasound?
Older patients with limb ischemia - an aortic aneurysm can have atherosclerosis or a mural thrombus which can embolize and cause an arterial occlusion!
“But they fixed my aorta!” Aortic endograft leakage can sometimes present with symptoms that are similar to a AAA rupture, such as back pain, flank pain, or hemodynamic instability.
How to scan like a Pro
Always Start Smart: Aortic ultrasound can be tricky because of factors that seem out of our control, such as bowel gas or patient body habitus.
When scanning for an abdominal aortic aneurysm, start scanning in the epigastric region with a transverse view and apply constant pressure, gently pushing the bowel gas out of the way as you slide the probe down towards the patient’s feet.
Tell your patients to bend their knees! This relaxes the abdominal musculature and can help you move bowel gas or make better contact with the probe.
What if you still can’t see it? Try looking in the right upper quadrant view of the FAST exam!
Start with your probe in the right mix-axillary line and use the liver as your acoustic window. You may need to fan anteriorly or posteriorly depending on the patient’s body habitus and your positioning.
Unfortunately, this view predominantly visualizes the superior aspect of the abdominal aorta, and it can be difficult to visualize the inferior abdominal aorta or the bifurcation.
Here we are looking at a modified RUQ view, where the aorta is visualized on the bottom part of the screen using the liver as an acoustic window. (acep.org)
Pro Pickups!
What’s that weird aneurysm?
Most people are familiar with the classic fusiform aortic aneurysm, but saccular aneurysms can be easily missed because of shadowing bowel gas obstructing parts of the aorta. Saccular aneurysms actually have a higher risk of rupture and repair is recommended for smaller diameters.
Here you can see two images in the longitudinal axis of the different kinds of abdominal aortic aneurysms. On the left is a saccular aneurysm and on the right is a fusiform one. Be sure to pay attention to the mural thrombus in the walls of both of these aortas - they can embolize and cause arterial occlusions! (med.emory.edu)
2. How big is that aorta anyways?
Be sure to always measure the aorta from outside wall to outside wall!
Many aortic aneurysms have a mural thrombus or intraluminal clot, and it can be very easy to mistake these for extra-luminal contents.
Remember the concerning numbers: >5.5cm for men and >5cm for women!
What the Pros Do Next
Abdominal Aortic Aneurysm
If the patient is hemodynamically unstable (defined as BP <90/60, altered mental status, or other signs of end-organ damage), go straight to the OR!
If the patient is hemodynamically stable (defined as the absence of any of the above), then the next step is to obtain further imaging, such as a CT Angiogram, which is the imaging gold standard.
If you are concerned about a large AAA that could be a contained leak but the patient is hemodynamically stable, then we recommend an emergent vascular surgery consult
If you find a small AAA (defined as <5cm in women or <5.5cm in men) that you do not think is actively contributing to the patient’s symptoms, then we recommend outpatient vascular surgery follow up
SonoPro Tips - Where to Learn More
Do you want to review more examples of pathologic images that you may see when you are doing an aortic ultrasound? Be sure to check out The Pocus Atlas by our expert editor Dr. Macias. Aortic pathology is quite rare, and going through these images will help immensely in recognizing this diagnosis in emergent situations. If you’re interested in looking at some of the evidence behind aortic ultrasound, be sure to check out the evidence atlas here as well.
References
Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013 Feb;20(2):128-38. doi: 10.1111/acem.12080. PMID: 23406071.
Hunter-Behrend, Michelle, and Laleh Gharahbaghian. “American College of Emergency Physicians.” ACEP // Home Page, 2016, www.acep.org/how-we-serve/sections/emergency-ultrasound/news/february-2016/tips-and-tricks-big-red---the-aorta-and-how-to-improve-your-image/.
Ma, John, et al. Ma and Mateer's Emergency Ultrasound. McGraw-Hill Education, 2020.
Mallin, Mike, and Matthew Dawson. Introduction to Bedside Ultrasound: Volume 1. Emergency Ultrasound Solutions, 2013.
Macias, Michael. TPA, www.thepocusatlas.com/.
Expert Commentary
Another great Sono Pro Post! Thank you John Li and Andra for helping everyone move from good to great when scanning for Abdominal Aortic Aneurysms. As noted, this application defines Emergency Ultrasound as a fast (pun intended), accurate, and life saving diagnostic tool for every EM physicians tool belt. When consistent probe pressure does not do the trick, consider the RUQ view for a quick look. Since most AAA’s are fusiform, this may quickly confirm your suspicions and prompt the call to get the OR ready. Be sure to visualize the entire abdominal aorta throughout in both short and long axis to identify saccular aneurysms and even the rare aortic occlusion!
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Li, J. Farcas, A. (2021 Oct 11). SonoPro Tips and Tricks for Aortic Aneurysm. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-aortic-aneurysm
Other Posts You May Enjoy
SonoPro Tips and Tricks for Pulmonary Embolism
Written by: Megan Chenworth, MD (NUEM ‘24) Edited by: Abiye Ibiebele, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Did you know that focused transthoracic cardiac ultrasound (FOCUS) can help identify PE in tachycardic or hypotensive patients? (It has been shown to have a sensitivity of 92% for PE in patients with an HR>100 or SBP<90, and approaches 100% sensitivity in patients with an HR>110 [1]). Have a hemodynamically stable patient with PE and wondering how to risk stratify? FOCUS can identify right heart strain better than biomarkers or CT [2].
Who to FOCUS on?
Patients presenting with chest pain or dyspnea without a clear explanation, or with a clinical concern for PE. The classic scenario is a patient with pleuritic chest pain with VTE risk factors such as recent travel or surgery, systemic hormones, unilateral leg swelling, personal or family history of blood clots, or known hypercoagulable state (cancer, pregnancy, rheumatologic conditions).
Patients presenting with unexplained tachycardia or dyspnea with VTE risk factors
Unstable patients with undifferentiated shock
When PE is suspected but CT is not feasible: such as when the patient is too hemodynamically unstable to be moved to the scanner, too morbidly obese to fit on the scanner, or in resource-limited settings where scanners aren’t available
One may argue AKI would be another example of when CT is not feasible (though there is some debate over the risk of true contrast nephropathy - that is a discussion for another blog post!)
How to scan like a Pro
Key is to have the patient as supine as possible - this may be difficult in truly dyspneic patients
If difficulty obtaining views arise, the left lateral decubitus position helps bring the heart closer to the chest wall
FOCUS on these findings
You only need one to indicate the presence of right heart strain (RHS).
Right ventricular dilation
Septal flattening: Highly specific for PE (93%) in patients with tachycardia (HR>100) or hypotension (SBP<90) [1]
Tricuspid valve regurgitation
McConnell’s sign
Definition: Akinesis of mid free wall and hypercontractility of apical wall (example below)
The most specific component of FOCUS: 99% specific for patients with HR>100bpm or SBP<90 [1]
Tricuspid annular plane systolic excursion (TAPSE)
The most sensitive single component of FOCUS: TASPE < 2cm is 88% sensitive for PE in tachycardic and hypotensive patients; 93% sensitive when HR > 110 [1]
Where to FOCUS
Apical 4 Chamber (A4C) view: your best shot at seeing it all
Find the A4C view in the 5th intercostal space in the midclavicular line
Optimize your image by sliding up or down rib spaces, sliding more lateral towards the anterior axillary line until you see the apex with the classic 4 chambers - if the TV and MV are out of the plane, rotate the probe until you can see both openings in the same image; if the apex is not in the middle of the screen, slide the probe until the apex is in the middle of the screen. If you are having difficulty with this view, position the patient in the left lateral decubitus.
Important findings:
RV dilation: the normal RV: LV ratio in diastole is 0.6:1. If the RV > LV, it is abnormal. (see in the image below)
Septal flattening/bowing is best seen in this view
McConnell’s sign: akinesis of the free wall with preserved apical contractility
McConnell’s Sign showing akinesis of the free wall with preserved apical contractility
4. Tricuspid regurgitation can be seen with color flow doppler when positioned over the tricuspid valve
Tricuspid regurgitation seen with color doppler flow
5. TAPSE
Only quantitative measurement in FOCUS, making it the least user-dependent measurement of right heart strain [3]
A quantitative measure of how well the RV is squeezing. RV squeeze normally causes the tricuspid annulus to move towards the apex.
Fan to bring the RV as close to the center of the screen as possible
Using M-mode, position the cursor over the lateral tricuspid annulus (as below)
Activate M-mode, obtaining an image as below
Measure from peak to trough of the tracing of the lateral tricuspid annulus
Normal >2cm
How to measure TAPSE using ultrasound
Parasternal long axis (PSLA) view - a good second option if you can’t get A4C
Find the PSLA view in the 4th intercostal space along the sternal border
Optimize your image by sliding up, down, or move laterally through a rib space, by rocking your probe towards or away from the sternum, and by rotating your probe to get all aspects of the anatomy in the plane. The aortic valve and mitral valve should be in plane with each other.
Important findings:
RV dilation: the RV should be roughly the same size as the aorta and LA in this view with a 1:1:1 ratio. If RV>Ao/LA, this indicates RHS.
Septal flattening/bowing of the septum into the LV (though more likely seen in PSSA or A4C views)
Right heart strain demonstrated by right ventricle dilation
Parasternal Short Axis (PSSA) view: the second half of PSLA
Starting in the PSLA view, rotate your probe clockwise by 90 degrees to get PSSA
Optimize your image by fanning through the heart to find the papillary muscles - both papillary muscles should be in-plane - if they are not, rotate your probe to bring them both into view at the same time
Important findings:
Septal flattening/bowing: in PSSA, it is called the “D-sign”.
“D-sign” seen on parasternal short axis view. The LV looks like a “D” in this view, particularly in diastole.
Subxiphoid view: can add extra info to the FOCUS
Start just below the xiphoid process, pointing the probe up and towards the patient’s left shoulder
Optimize your image by sliding towards the patient’s right, using the liver as an echogenic window; rotate your probe so both MV and TV are in view in the same image
Important findings
Can see plethoric IVC if you fan down to IVC from RA (not part of FOCUS; it is sensitive but not specific to PE)
Plethoric IVC that is sensitive to PE
What to do next?
Sample algorithm for using FOCUS to assess patients with possible PE.
*cannot completely rule out PE, but negative FOCUS makes PE less likely
Limitations to keep in mind:
FOCUS is great at finding heart strain, but the lack of right heart strain does not rule out a pulmonary embolism
Systematic review and meta-analysis concluded that the overall sensitivity of FOCUS for PE is 53% (95% CI 45-61%) for all-comers [5]
Total FOCUS exam requires adequate PSLA, PSSA, and A4C views – be careful when interpreting inadequate scans
Can see similar findings in chronic RHS (pHTN, RHF)
Global thickening of RV (>5mm) can help distinguish chronic from acute RHS
McConell’’s sign is also highly specific for acute RHS, whereas chronic RV failure typically appears globally akinetic/hypokinetic
SonoPro Tips - Where to Learn More
Right Heart Strain at 5-Minute Sono: http://5minsono.com/rhs/
Ultrasound GEL for Sono Evidence: https://www.ultrasoundgel.org/posts/EJHu_SYvE4oBT4igNHGBrg, https://www.ultrasoundgel.org/posts/OOWIk1H2dePzf_behpaf-Q
The Pocus Atlas for real examples: https://www.thepocusatlas.com/echocardiography-2
The Evidence Atlas for Sono Evidence: https://www.thepocusatlas.com/ea-echo
References
Daley JI, Dwyer KH, Grunwald Z, Shaw DL, Stone MB, Schick A, Vrablik M, Kennedy Hall M, Hall J, Liteplo AS, Haney RM, Hun N, Liu R, Moore CL. Increased Sensitivity of Focused Cardiac Ultrasound for Pulmonary Embolism in Emergency Department Patients With Abnormal Vital Signs. Acad Emerg Med. 2019 Nov;26(11):1211-1220. doi: 10.1111/acem.13774. Epub 2019 Sep 27. PMID: 31562679.
Weekes AJ, Thacker G, Troha D, Johnson AK, Chanler-Berat J, Norton HJ, Runyon M. Diagnostic Accuracy of Right Ventricular Dysfunction Markers in Normotensive Emergency Department Patients With Acute Pulmonary Embolism. Ann Emerg Med. 2016 Sep;68(3):277-91. doi: 10.1016/j.annemergmed.2016.01.027. Epub 2016 Mar 11. PMID: 26973178.
Kopecna D, Briongos S, Castillo H, Moreno C, Recio M, Navas P, Lobo JL, Alonso-Gomez A, Obieta-Fresnedo I, Fernández-Golfin C, Zamorano JL, Jiménez D; PROTECT investigators. Interobserver reliability of echocardiography for prognostication of normotensive patients with pulmonary embolism. Cardiovasc Ultrasound. 2014 Aug 4;12:29. doi: 10.1186/1476-7120-12-29. PMID: 25092465; PMCID: PMC4126908.
Hugues T, Gibelin PP. Assessment of right ventricular function using echocardiographic speckle tracking of the tricuspid annular motion: comparison with cardiac magnetic resonance. Echocardiography. 2012 Mar;29(3):375; author reply 376. doi: 10.1111/j.1540-8175.2011.01625_1.x. PMID: 22432648.
Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta‐analysis. J Am Soc Echocardiogr 2017;30:714–23.e4.
Expert Commentary
RV function is a frequently overlooked area on POCUS. Excellent post by Megan looking specifically at RV to identify hemodynamically significant PEs. We typically center our image around the LV, so pay particular attention to adjust your views so the RV is optimized. This may mean moving the footprint more laterally and angle more to the patient’s right on the A4C view. RV: LV ratio is often the first thing you will notice. When looking for a D-ring sign, make sure your PSSA is actually in the true short axis, as a diagonal cross-section may give you a false D-ring sign. TAPSE is a great surrogate for RV systolic function as RV contracts longitudinally. Many patients with pulmonary HTN or advanced chronic lung disease can have chronic RV failure, lack of global RV thickening. Lastly remember, that a positive McConnell’s sign is a great way to distinguish acute RHS from chronic RV failure.
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Chenworth, M. Ibiebele, A. (2021 Oct 4). SonoPro Tips and Tricks for Pulmonary Embolism. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-pulmonary-embolism
Other Posts You May Enjoy
SonoPro Tips and Tricks for Pneumothroax
Written by: Morgan McCarthy, MD (NUEM ‘24) Edited by: Jon Hung, MD (NUEM ‘21) Expert Commentary by: John Bailitz, MD & Shawn Luo, MD (NUEM ‘22)
SonoPro Tips and Tricks
Welcome to the NUEM Sono Pro Tips and Tricks Series where Sono Experts team up to take you scanning from good to great for a problem or procedure! For those new to the probe, we recommend first reviewing the basics in the incredible FOAMed Introduction to Bedside Ultrasound Book and 5 Minute Sono. Once you’ve got the basics beat, then read on to learn how to start scanning like a Pro!
Did you know that Lung Ultrasound (LUS) has a higher sensitivity than the traditional upright anteroposterior chest X-ray for the detection of a pneumothorax? (LUS has a reported 90.9 for sensitivity and 98.2 for specificity. CXR were 50.2 for sensitivity and 99.4 for specificity). Busy trauma bay? Ultrasound is faster than calling for X-ray. Critically ill patient? Small pneumothoraces are less likely to be missed with ultrasound. To take your Sono Skills to the next level, read on:
Beyond the classic trauma patient during your E-Fast Exam, who else does the Sono-Pros scan?
Primary spontaneous pneumothorax: the classic scenario is a tall, young adult, with symptoms such as breathlessness, along with potentially those with risk factors of pneumothoraxes such as smoking, male sex, family history of pneumothorax
Secondary spontaneous pneumothorax: those with underlying lung disease including but not limited to COPD, tuberculosis, necrotizing pneumonia, pneumonocystis carini, lung cancer, sarcoma involving the lung, sarcoidosis, endometriosis, cystic fibrosis, acute severe asthma, idiopathic pulmonary fibrosis
Of course, traumatic pneumothorax, especially in penetrating trauma or blunt trauma with broken ribs
Don’t forget iatrogenic causes of pneumothorax including transthoracic needle aspiration, subclavian vessel puncture, thoracentesis, pleural biopsy, and mechanical ventilation
SonoPro Tips - How to scan like a Pro
The key is to have the patient completely supine - air rises! - with the probe in the anterior field in sagittal orientation pointing towards the patient's head.
It is commonly taught to start at the second intercostal space, midclavicular line, and scan down a few lung spaces to at least the 4th intercostal space, however, keep in mind some studies show that trauma supine trauma patients had pneumothoraces seen more commonly in the 5-8 rib spaces.
Important Landmarks
Green = Subcutaneous tissue. Red = Pleural space. Blue = A - lines.
4. Look for lung sliding, improve your image by turning down gain and decrease depth to have lung sliding become clearer
What to Look For:
To Rule-Out a pneumothorax
Lung Sliding - Lung sliding has a negative predictive value of 100% for ruling out a pneumothorax, however only at that interspace
Additional Findings: B-lines and Z lines also help to rule out pneumothorax!
2. To Rule-In a pneumothorax
Lung point - the interface between where lung sliding is happening and where the absence of lung sliding is happening has been shown to have 100% specificity for pneumothorax.
Keep in mind the border of where the heart and lung come in contact and the border where the diaphragm and lung come in contact can cause a false lung point.
The lung point may be hard to find in a larger pneumothorax, and impossible to find in a completely collapsed lung.
3. Next turn on M-mode:
Sandy Beach Shore = Lung sliding (left). Barcode Sign = No lung sliding (right)
What to do next:
Lung sliding = sensitive, Lung point = specific
If you see lung sliding, there is no pneumothorax
If you do not see lung sliding it does not rule in a pneumothorax -> look for a lung point, the interface between where lung sliding is happening and where the absence of lung sliding is happening to rule it in
Always keep in mind other causes that result in lack of lung sliding before management decisions take place!: atelectasis, main-stem intubation, adhesions, contusions, and arrest or apnea. Check out this great table from 5 - Min Sono.
4. If your patient is apneic or has a mainstem intubation look for lung pulse, when the heart beats if the parietal and visceral pleura are touching (no pneumothorax) it will show a pulse at the interfaces of the pleura
5. Sub-Q emphysema - Always look for E - lines. When there is subcutaneous air above the pleural line it creates a false pleural line above the actual pleural. You may also see B-lines obscuring the actual pleural line. This is most likely subcutaneous air and you can not interpret it for a pneumothorax.
SonoPro Tips - Where to Learn More
American College of Emergency Physicians. Emergency ultrasound imaging criteria compendium. Ann Emerg Med. 2006;48(4):487-510.
Ma, John, et al. Ma and Mateer's Emergency Ultrasound. McGraw-Hill Education, 2020.
Macias, Micheal. TPA, The Pocus Atlas.
Availa, Jacob. 5 minute Sono.
Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708.
Expert Commentary
Morgan went “beyond lung sliding” and dove deep into how to increase your sensitivity & specificity for PTX with POCUS. Supine is ideal to make PTX visible against the anterior chest wall, but if the patient cannot tolerate lying flat, look at the apical pleural superior to the clavicles. First, identify the true pleural line--it should be the bright line just deep to the ribs in your view. SQ emphysema may obscure the view or even mimic the pleura, although its outline is usually more hazy & irregular, a little pressure helps to move the SQ air out of the way can be helpful. Sliding? Great, PTX ruled out. But absent sliding does not automatically mean PTX. Make sure there is no B-line or “lung pulse”, as sometimes pleural adhesion or poor ventilation can cause absent sliding too. Most of the time you don’t need M-mode unless the movement is very subtle and you want to be extra sure. The lung point is pathognomonic for PTX, but don’t waste time digging around for it if the patient is unstable with a good clinical story for PTX > decompress instead!
John Bailitz, MD
Vice Chair for Academics, Department of Emergency Medicine
Professor of Emergency Medicine, Feinberg School of Medicine
Northwestern Memorial Hospital
Shawn Luo, MD
PGY4 Resident Physician
Northwestern University Emergency Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] McCarthy, M. Hung J. (2021 Sept 20). SonoPro Tips and Tricks for Pneumothorax. [NUEM Blog. Expert Commentary by Bailitz, J. Shawn, L.]. Retrieved from http://www.nuemblog.com/blog/sonopro-tips-and-tricks-for-pneumothorax
Other Posts You May Enjoy
Hanging Injuries
Written by: Vytas Karalius, MD, MPH (NUEM ‘22) Edited by: Nery Porras, MD (NUEM ‘21) Expert Commentary by: Kevin Emmerich, MD, MS
Today’s post was inspired by the near-hanging of young gentleman who ended up passing away due to complications related to his near-hanging. His parents decided to donate his organs to Gift of Hope, allowing the passing of his life to extend the lives of others. While we hope to never see cases like these, they are an inevitable part of our job as emergency medicine physicians. As with most rare and complex pathology, preparation and knowledge can help us with the management of these cases when things often get chaotic. Lastly, as emergency medicine physicians who see the sequelae of mental illness daily in their EDs, I encourage us all to advocate for better funding and access to mental health care in the United States.
Hanging Injury
Terms/Classification [1]
“Hanging” is used to describe a death from a form of strangulation that involves hanging from the neck.
“Near-hanging” is a term for patients who have survived an attempted hanging (or at least long enough to reach the hospital).
“Complete hanging” defines when a patient’s legs are fully suspended off the ground and the patient's bodyweight is fully suspended by the neck.
“Incomplete hanging” defines when some part of the patient’s body is still on the ground and the body's full weight is not suspended off the ground.
“Judicial hanging” classically refers to victims who fell at least the height of their body.
Epidemiology:
Hanging is the 2nd most common form of successful suicide in the US after firearms
Accounts for 23% of >34,500 suicides in 2007
In the jail system, hanging is the most common form of successful suicide
Increasing incidence in US
Risk Factors: male, aged 15-44 years, history of drug or alcohol abuse, history of psychiatric illness
Pathophysiology of Injury:
Spine/Spinal Cord:
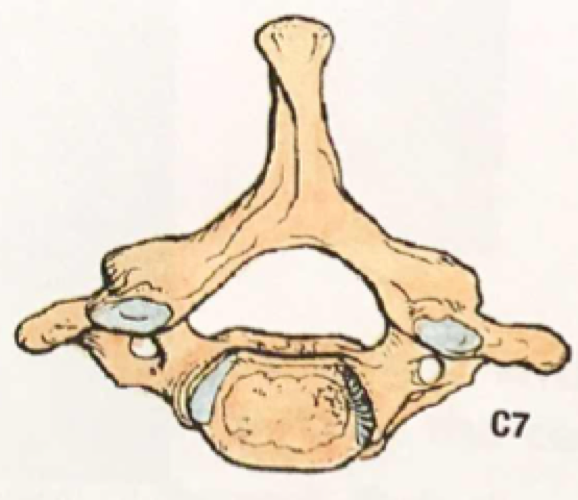
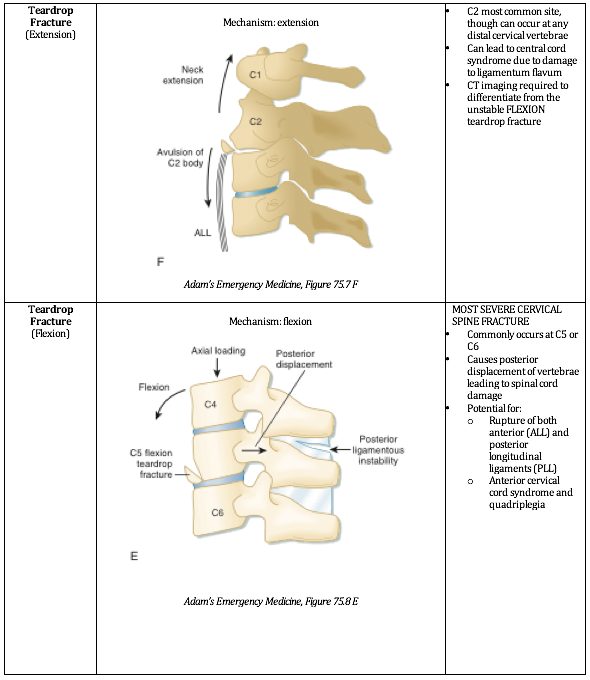
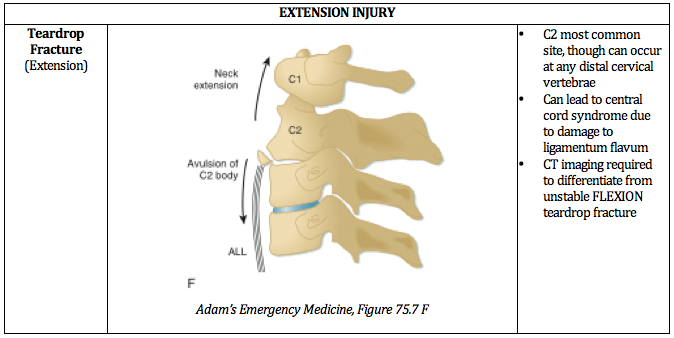
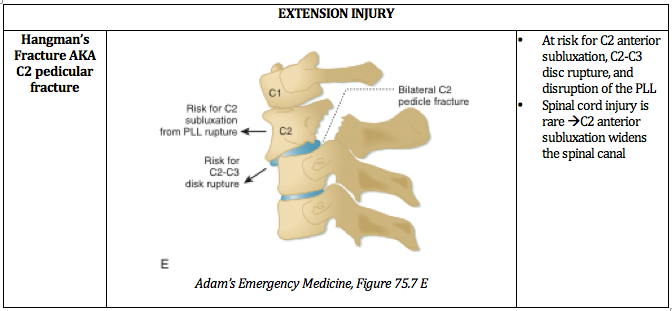
When the drop is greater than or equal to the height of the victim, as in a judicial hanging, there will almost always be cervical spine injury.
The head hyperextends, leading to fracture of the upper cervical spine ("hangman's fracture” of C2) and transection of the spinal cord.
Cervical injuries are in non-judicial hangings are rare. [2] One retrospective case review of near-hangings over a 10-year period found the incidence of cervical spine fracture to be as low as 5%. [3]
Vascular:
The major pathologic mechanism of death in hanging/strangulation is neck vessel occlusion, not airway obstruction. [1,4]
Death ultimately results from cerebral hypoxia and global ischemia.
There are two mechanisms by which this happens:
Venous: The most implicated cause of death is actually venous obstruction. Jugular veins are superficial and easily compressible. Obstruction of venous outflow from the brain leads to stagnant hypoxia and loss of consciousness in as little as 15 seconds.
Arterial: The risk of damage to the major arterial blood flow to the brain (such as carotid artery dissection) is rare, but should suspected in patients. [4]
Cardiac:
Carotid body reflex-mediated cardiac dysrhythmias are reported, and likely a minor mechanism of death.
Pulmonary:
Airway compromise plays less of a role in the immediate death of complete hanging/strangulation. However, it is a major cause of delayed mortality in near-hanging victims. [1,4]
Significant pulmonary edema occurs through two mechanisms:
Neurogenic: centrally mediated, massive sympathetic discharge; often in association with serious brain injury and a poor prognostic implication.
Post-obstructive: strangulation causes marked negative intrapleural pressure, generated by forceful inspiratory effort against extra-thoracic obstruction; when the obstruction is removed, there is a rapid onset pulmonary edema leading to ARDS.
Aspiration pneumonia later sequela of near-hanging injury.
Airway edema from mechanical trauma to the airway, which can make intubation difficult.
Tracheal stenosis can develop later in the hospital course.
Other Injuries:
Hyoid bone fracture
Cricoid or thyroid cartilage injury [5]
Physical Examination:
"Ligature marks" or abrasions, lacerations, contusions, bruising, edema of the neck
Tardieu spots of the eyes
Severe pain on gentle palpation of the larynx (laryngeal fracture)
Respiratory signs: cough, stridor, dysphonia/muffled voice, aphonia
Varying levels of respiratory distress
Hypoxia
Mental status changes
Early Management/Stabilization:
ABCs as always
Early endotracheal intubation may become necessary with little warning.
Patients who are unconscious or have symptoms such as odynophagia, hoarseness, neurologic changes, or dyspnea require aggressive airway management.
If ETI unsuccessful, consider cricothyroidotomy; if unsuccessful, percutaneous trans-laryngeal ventilation may be used temporarily.
Judicious and cautious fluid resuscitation - avoid large fluid volume resuscitation and consider early pressors, as fluids increases the risk/severity of ARDS and cerebral edema.
Monitor for cardiac arrhythmias.
The altered/comatose patient should be assumed to have cerebral edema with elevated ICP.
Imaging/Further Testing:
Chest radiograph
CT brain
CT C-spine
CTA head/neck
Can consider soft-tissue neck x-ray, if CT not immediately available
Further Management:
In patients with signs of hanging/strangulation, there should be a low threshold to obtain diagnostic imaging/testing as discussed above.
Expect pulmonary complications early.
They are a major cause of delayed mortality in near-hanging victims, as stated above.
Early intubation and airway management are important.
Non-intubated patients with pulmonary edema may benefit from positive end-expiratory pressure ventilation.
Patients with symptoms of laryngeal or tracheal injury (e.g. dyspnea, dysphonia, aphonia, or odynophagia), should undergo laryngobronchoscopy with ENT. [4,6]
Tracheal stenosis has been reported during the hospital course. Address cerebral edema from anoxic brain injury, using strategies to reduce intracranial pressure or seizure prophylaxis. [4]
Address vascular complications seen on CTA and coordinate intervention with the appropriate specialty at your institution.
Therapeutic Hypothermia
There is some evidence for therapeutic hypothermia in those with cardiac arrest from hanging injury [7,8] and those who are comatose from hanging injury. [9-11] While the evidence is weak, in the absence of better evidence, it is reasonable to consider hypothermia treatment in all comatose near-hanging victims. [1,12,13]
When suicide is suspected, evaluate patients for other methods of self-harm (e.g. wrist lacerations, self-stabbing, ingestions). It is also important to consider drug and alcohol intoxication. [4]
Disposition:
Admit critically ill patients to the appropriate ICU-level care.
Admit patients with abnormal radiologic or endoscopic imaging to the appropriate service and level of care.
Even if the initial presentation is clinically benign, all near-hanging victims should be observed for 24 hours, given the high risk of delayed neurologic, airway and pulmonary complications. [14]
Observe asymptomatic patients with normal imaging.
Psychiatry/Crisis Team consult on all suspected intentional cases.
Emphasize strict return precautions as well as education about possible delayed respiratory and neurologic dysfunction when discharging patients.
Some patients may require transfer to a trauma center if the required services are not available at the initial receiving facility. [1]
Prognostication:
GCS 3/GCS 3T is a predictor of very poor outcome, [15-19] but there is mixed evidence on the GCS as a predictor of outcomes in GCS scores greater than 3, especially with regard to neurologic intactness. [3,19]
Recovery of patients with neurology symptoms is unpredictable. [4]
Patients presenting with cardiac arrest have a very poor prognosis, and might be the strongest predictor of poor prognosis. [4,8,16,18,20]
Other predictors of poor clinical outcome include:
Anoxic brain injury or cerebral edema on head CT [3,19]
Prolonged hanging time [18]
Cardiopulmonary arrest [8,11,19]
Cervical spine injury
Hypotension on arrival
Expert Commentary
We’ve all certainly been involved with a patient with reported hanging injury at some point in our time in the ED. They are usually unimpressive if a person does it as more of a gesture rather than a true suicide attempt. When they are unfortunately done “correctly,” they usually result in a trip to the morgue instead of the ED. When the swiss cheese holes align and a true hanging attempt results in a serious but not fatal presentation, things can get quite hairy. I’ve been a part of one such case, and will never forget it. Here are my two cents.
Airway
This should undoubtedly be treated as a predicted difficult airway, not only due to likely cervical spine trauma, but also possibly due to airway edema. Get your ducks in a row for this unless this patient is crashing in front of you. Get your consultants/help (if available), preoxygenate, airway adjuncts open and ready, backup airway supplies if your first plan fails. Most importantly, have a plan and discuss this with your team beforehand. Don’t be afraid to take an awake look with a hyperangulated video laryngoscope, especially if this patient presents with stridor. Ketamine can be your friend here. This should be an airway that you do not undertake without a scalpel, finger, and bougie ready just in case. I like to draw a line on the patient’s skin overlying the cricothyroid membrane beforehand.
Trauma
Self explanatory, but don’t be stingy here. Light this patient up from head to pelvis, including the neck angiogram. Document a repeat neuro exam every time you move this patient.
Overdose/psych
Don’t forget your Tylenol and salicylate levels, EKG in this suicide attempt. If you feel the need to add the useless urine drug screen, I suppose this is fine as well.
Kevin Emmerich, MD, MS
Emergency Medicine Physician
Methodist Hospital
Gary, Indiana
How To Cite This Post:
[Peer-Reviewed, Web Publication] Karalius, V. Porras, N. (2021, Aug 9). Hanging Injuries. [NUEM Blog. Expert Commentary by Emmerich, K]. Retrieved from http://www.nuemblog.com/blog/hanging-emergencies
Other Posts You May Enjoy
References
1. Walls RM, Hockberger RS, Gausche-Hill M. Rosen's emergency medicine : concepts and clinical practice. Ninth edition. ed. Philadelphia, PA: Elsevier; 2018.
2. Aufderheide TP, Aprahamian C, Mateer JR, et al. Emergency airway management in hanging victims. Ann Emerg Med. 1994;24(5):879-884.
3. Salim A, Martin M, Sangthong B, Brown C, Rhee P, Demetriades D. Near-hanging injuries: a 10-year experience. Injury. 2006;37(5):435-439.
4. Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM. Tintinalli's emergency medicine: a comprehensive study guide. 9th. ed. New York: McGraw-Hill Education; 2019.
5. Tugaleva E, Gorassini DR, Shkrum MJ. Retrospective Analysis of Hanging Deaths in Ontario. J Forensic Sci. 2016;61(6):1498-1507.
6. Hackett AM, Kitsko DJ. Evaluation and management of pediatric near-hanging injury. Int J Pediatr Otorhinolaryngol. 2013;77(11):1899-1901.
7. Hsu CH, Haac B, McQuillan KA, Tisherman SA, Scalea TM, Stein DM. Outcome of suicidal hanging patients and the role of targeted temperature management in hanging-induced cardiac arrest. J Trauma Acute Care Surg. 2017;82(2):387-391.
8. Kim MJ, Yoon YS, Park JM, et al. Neurologic outcome of comatose survivors after hanging: a retrospective multicenter study. Am J Emerg Med. 2016;34(8):1467-1472.
9. Jehle D, Meyer M, Gemme S. Beneficial response to mild therapeutic hypothermia for comatose survivors of near-hanging. Am J Emerg Med. 2010;28(3):390.e391-393.
10. Lee BK, Jeung KW, Lee HY, Lim JH. Outcomes of therapeutic hypothermia in unconscious patients after near-hanging. Emerg Med J. 2012;29(9):748-752.
11. Hsu CH, Haac BE, Drake M, et al. EAST Multicenter Trial on targeted temperature management for hanging-induced cardiac arrest. J Trauma Acute Care Surg. 2018;85(1):37-47.
12. Borgquist O, Friberg H. Therapeutic hypothermia for comatose survivors after near-hanging-a retrospective analysis. Resuscitation. 2009;80(2):210-212.
13. Sadaka F, Wood MP, Cox M. Therapeutic hypothermia for a comatose survivor of near-hanging. Am J Emerg Med. 2012;30(1):251.e251-252.
14. McHugh TP, Stout M. Near-hanging injury. Ann Emerg Med. 1983;12(12):774-776.
15. Kao CL, Hsu IL. Predictors of functional outcome after hanging injury. Chin J Traumatol. 2018;21(2):84-87.
16. La Count S, Lovett ME, Zhao S, et al. Factors Associated With Poor Outcome in Pediatric Near-Hanging Injuries. J Emerg Med. 2019;57(1):21-28.
17. Martin MJ, Weng J, Demetriades D, Salim A. Patterns of injury and functional outcome after hanging: analysis of the National Trauma Data Bank. Am J Surg. 2005;190(6):836-840.
18. Matsuyama T, Okuchi K, Seki T, Murao Y. Prognostic factors in hanging injuries. Am J Emerg Med. 2004;22(3):207-210.
19. Nichols SD, McCarthy MC, Ekeh AP, Woods RJ, Walusimbi MS, Saxe JM. Outcome of cervical near-hanging injuries. J Trauma. 2009;66(1):174-178.
20. Gantois G, Parmentier-Decrucq E, Duburcq T, Favory R, Mathieu D, Poissy J. Prognosis at 6 and 12months after self-attempted hanging. Am J Emerg Med. 2017;35(11):1672-1676.
Scalpel Finger Bougie
Written by: Em Wessling, MD (NUEM ‘22) Edited by: Therese Whipple (NUEM ‘20) Expert Commentary by: Joseph Posluszny, MD
Expert Commentary
Establishing an airway via a cricothyroidotomy is a stressful and tense experience. In almost all of these cases, experienced airway staff have already attempted advanced airway maneuvers in patients typically at high risk for inability to intubate. As the oxygen saturation drops and the patient becomes unstable, the most adept proceduralist present (whether emergency department physicians or surgeons) are asked to step in to secure a surgical airway.
The scalpel-finger-bougie technique is one proven and reliable method to secure a surgical airway via a cricothyroidotomy. Some additions to the technique described above are:
Use a vertical incision through the skin and soft tissues. If you are too superior or inferior with your initial incision, then this incision can be easily extended as needed. A horizontal incision commits you to that cranial-caudal level. It is often more of a struggle to identify the cranial-caudal orientation of the cricothyroid membrane rather than the medial-lateral orientation.
In a patient with a stable and flexible neck, retract the neck via cranial pressure on the chin to bring the neck structures better into your working field. Insert a shoulder roll if available (unlikely) to augment this positioning.
After the tube is advanced, listen for bilateral breath sounds. It is common, in this adrenaline fueled procedure, to advance the endotracheal tube too far, leading to a right main stem intubation. This can limit your ventilation and oxygenation and can lead to confusion about the airway placement in the neck. If there are no left lung field breath sounds, then pull the tube back until bilateral breath sounds are confirmed with auscultation.
Always verify tube placement with capnography.
Persistent, moderate volume bleeding is often from injury to the anterior jugular vein. Gentle, directed pressure on the area can control this bleeding while the patient is being transported to the operating room for a more definitive airway.
Joseph Posluszny, MD
Assistant Professor of Surgery (Trauma and Critical Care)
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Wessling, E. Whipple, T. (2021, Aug 2). Scalpel Finger Bougie. [NUEM Blog. Expert Commentary by Posluszny, J]. Retrieved from http://www.nuemblog.com/blog/scalpel-finger-bougie
Other Posts You May Enjoy
Elderly Fallers
Written by: Nick Wleklinski, MD (NUEM ‘22) Edited by: Kumar Gandhi, MD, MPH (NUEM '20) Expert Commentary by: Scott Dresden, MD, MS
Oh How the Older Adults Fall
Introduction:
Older adults (>65yrs old) fall. In 2006, older adult patients who fell made up approximately 2.1 million of ED visits totaling $6.1 billion in health care dollars [1]. Falls are the most common cause of unintentional injury for older folks, accounting for 13% of all ED visits from 2008-2010 [2]. These numbers are only increasing as our population ages and it is predicted to double by 2030 [3]. The injuries incurred wildly vary, but these patients tend to fall into two buckets: Major injury/organic etiology à admit vs. simple mechanical fall à Discharge.
Common injuries requiring hospitalization:
Falls resulting in major injury carry significant morbidity and mortality. Hip fractures lead to deterioration in function and carry ~27% mortality at 1 year [4]. Head injuries account for a significant amount of fall-related deaths, making CT brain imagining imperative in most fall patients. Add a CT C-spine as these injuries are more common in the older adults, the Canadian and Nexus C-spine rules don’t work well for these patients [5]. Additionally, rib fractures are common and require significant analgesia to prevent splinting and subsequent complications. Be sure to consider blunt cardiac injury and pulmonary contusion! Given that falls are a frequent cause of trauma in older adult patients, it is important to keep the effects of aging in mind when running the ABC’s (Table 1) [6].
Table 1: Further considerations for ABC’s in older adult trauma patients.
The tougher scenario: Those without any injuries:
Patients without any major injuries deserve more thought than simply ruling out organic etiologies (i.e. CVA, ACS, arrythmia, etc.) and major trauma. These patients are at high risk for subsequent falls and may even have underlying physiologic injuries. Using the term “mechanical fall” is risky as it can anchor providers into comfort. Therefore, having a more regimented approach can help better risk stratify these patients.
The fall itself:
Where did it happen?
Those in nursing homes/institutional setting fall more frequently than those in the community (60% vs ~33%, respectively) [7]
Falls at home should trigger need for home safety evaluation
Have you fallen before?
History tends to repeat itself, with nearly 50% of fallers falling again within 1 year [8]
Witnessed vs Unwitnessed?
Collateral information can provide key details if a patient is a fall risk and requires further evaluation by physical therapy
How long where you on the ground? [9, 10]
Figure 1: Increased time on the ground leads to worsening fall anxiety and increased risk of rhabdomyolysis and subsequent kidney injury
Evaluating the patient:
Outside of the obvious (CVA, ACS, etc.), it is important to also consider other common etiologies:
Hypotension
Arrythmias
Infection (PNA, UTI, pressure ulcers)
Vestibular dysfunction (i.e. BPPV)
Anemia
Ask about melena as this is a commonly not investigated [11]
Delirium
Malignancy
Medications: Polypharmacy is a known issue in older adults, but there are certain medications to take note of. Antidepressants and antipsychotics are associated with the highest risk of falls while diuretics and narcotics didn’t have as much of a risk (Table 2) [12]. Additionally, who manages the meds and how are they organized at home?
Table 2: Common medications associated with falls
What is their baseline? This is the meat and potatoes of the evaluation and where future risk factors can be identified and addressed.
How steady do they feel on their feet?
Decreased cognition (Dementia, Alzheimer’s, etc.) incurs increase fall risk [13]
Do they have arthritis/chronic pain?
Can result in unsteady gait from favoring certain part of body, increasing risk
Timed Up and Go Test:
A great way to evaluate lower extremity strength and balance (figure 2)
Visual and auditory impairment: Visual acuity should be addressed. Look at their eyewear as multifocal lenses increase fall risk [14].
Feet: check for neuropathy and ask about footwear.
Assist devices used for ambulation? Do they use these devices regularly and correctly?
Delirium screening
The Confusion Assessment Method is used in triage [15]
Figure 2: The Timed Up and Go Test.
Things we can do:
Although continuity is not generally part of the EM specialty, we can help address future fall risk for these patients who we discharge after their fall evaluation. Recommending supplements such as vitamin D and calcium are helpful for reducing risk for fall-related injuries [7]. Balance training through outpatient physical therapy referrals can further help reduce fall risk. Follow up is imperative and these patients should see their PMD or a geriatrician soon after their discharge from the ER to continue their fall evaluation.
Conclusion:
While major trauma from falls is exciting and straight forward, it is important to give more thought to those older adult patients deemed to have a “mechanical fall”. Gathering information about the fall and determining the patient’s baseline can help stratify future risk. The incidence of falls is only going to increase as our population ages, so having a regimented approach to these patients is imperative.
Expert Commentary
As was expertly described, falls in older adults lead to significant morbidity and mortality. Unfortunately, in the ED they are often dismissed as “mechanical,” the injuries are treated, but the causes are never identified. The term mechanical fall is ambiguous and unhelpful and should not be used in the ED. Some mean that the fall was not a result of seizure or syncope, but it is not a clear term. Additionally, it does not help with prognosis. There are no differences in adverse events at 6 months between “mechanical” and non-mechanical faller. For a great discussion of the Myth of the Mechanical Fall see Shan Liu’s presentation at IGNITE presentation at SAEM18 (https://saem-ondemand.echo360.org/media-player.aspx/5/13/431/1608).
Even if injuries are minor, patients often do poorly. Between 36% and 50% of patients have an adverse event such as a recurrent fall, emergency department revisit, or death within 1 year after a fall, including 25% who die within 1 year. As the CDC likes to remind us, every 20 minutes someone dies from a fall (https://www.cdc.gov/steadi/index.html).
So what do we do with this medical problem that has a 25% 1-year mortality? As with many problems in geriatrics, falls are a sentinel event, and deserve a sentinel response. It is our job to prevent the next fall. The Geriatric Emergency Department Guidelines provide a framework for a risk assessment after a fall. One might think that the cause of the fall is obvious (e.g. tripped over a crack in the sidewalk). However a thoughtful assessment begins by asking “if this patient was a health 20-year-old, would he or she have fallen? “ If the answer is no, then the assessment of the underlying cause of the fall should be more comprehensive and should include a thorough history of the fall and risk factors such as ability to perform Activities of Daily Living (ADLs), appropriate footwear, and medications. Physical exam should include orthostatic blood pressure, a head to toe exam even for patients with seemingly isolated injuries, a neurologic exam with special attention to neuropathy and proximal motor strength, and a safety assessment. Patients should be able to rise from the bed or chair, turn, and steadily ambulate in the ED before considering discharge (not while the nurse is handing the patient his or her discharge paperwork). For patients who are unable to safely ambulate, consideration of an assist device such as a cane or walker should be given, physical therapy (PT) and occupational therapy (OT) consultation, and possibly hospital admission. All patients who are admitted after a fall should be admitted by PT and OT. Additionally, patients who fell should have home safety assessments which may be arranged through occupational therapy.
In addition to the GED guidelines, the CDC has developed the Stopping Elderly Accidents, Deaths & Injuries (STEADI) program. This program includes an algorithm for fall risk screening, assessment and intervention. For screening they recommend patients answer the Stay Independent screening (a 12 question tool), however if the patient is in the ED for a fall, this step can be omitted because the patient has already declared themselves as high risk for falls. To evaluate gait, strength, and balance, the Timed Up & Go, 30-Second Chair Stand, or 4-Stage Balance Test are recommended. In addition, to the assessments mentioned previously (medications, orthostatics), asking about potential hazards such as throw rugs or slippery floors, and a visual acuity check are advised. Once risk factors are identified they should be addressed through physical therapy, exercise of fall prevention programs, medication optimization, home safety evaluation, discussion with outpatient clinicians regarding orthostatic hypotension, referral to a podiatrist for proper footwear, recommending a vitamin D supplement. Finally, ensuring close and enduring followup is important. Consider a referral to a geriatrician if the patient doesn’t already see one.
Scott Dresden, MD, MS
Associated Professor of Emergency Medicine
Director of Geriatric Emergency Department Innovations (GEDI)
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Wleklinski, N. Gandhi, G. (2020, Nov 23). Elderly Fallers. [NUEM Blog. Expert Commentary by Dresden, D]. Retrieved from http://www.nuemblog.com/blog/elderly-falls.
Other Posts You May Enjoy
References
Owens, P.L., et al., Emergency Department Visits for Injurious Falls among the Elderly, 2006: Statistical Brief #80, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006, Agency for Healthcare Research and Quality (US): Rockville (MD).
Sapiro, A.L., et al., Rapid recombination mapping for high-throughput genetic screens in Drosophila. G3 (Bethesda), 2013. 3(12): p. 2313-9.
Foundation, C.f.D.C.a.P.a.T.M.C. The State of Aging and Health in America 2007. 2007 [cited 2019.
Cenzer, I.S., et al., One-Year Mortality After Hip Fracture: Development and Validation of a Prognostic Index. J Am Geriatr Soc, 2016. 64(9): p. 1863-8.
Goode, T., et al., Evaluation of cervical spine fracture in the elderly: can we trust our physical examination? Am Surg, 2014. 80(2): p. 182-4.
Carpenter, C.R., et al., Major trauma in the older patient: Evolving trauma care beyond management of bumps and bruises. Emerg Med Australas, 2017. 29(4): p. 450-455.
Nagaraj, G., et al., Avoiding anchoring bias by moving beyond 'mechanical falls' in geriatric emergency medicine. Emerg Med Australas, 2018. 30(6): p. 843-850.
Liu, S.W., et al., Frequency of ED revisits and death among older adults after a fall. Am J Emerg Med, 2015. 33(8): p. 1012-8.
Austin, N., et al., Fear of falling in older women: a longitudinal study of incidence, persistence, and predictors. J Am Geriatr Soc, 2007. 55(10): p. 1598-603.
Deshpande, N., et al., Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc, 2008. 56(4): p. 615-20.
Tirrell, G., et al., Evaluation of older adult patients with falls in the emergency department: discordance with national guidelines. Acad Emerg Med, 2015. 22(4): p. 461-7.
Woolcott, J.C., et al., Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med, 2009. 169(21): p. 1952-60.
Muir, S.W., K. Gopaul, and M.M. Montero Odasso, The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing, 2012. 41(3): p. 299-308.
Lord, S.R., J. Dayhew, and A. Howland, Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc, 2002. 50(11): p. 1760-6.
Han, J.H., et al., Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med, 2013. 62(5): p. 457-465.
Eyelid Lacerations
Written by: Maurice Hajjar, MD (PGY-2) Edited by: Jessica Bode, MD (NUEM ‘19) Expert Commentary by: Rehan Hussain
Expert Commentary
Thank you for this excellent diagram, which demonstrates a thorough and systematic approach to eyelid lacerations encountered in the Emergency Room. I have a few extra pearls that may aid ER physicians in management of eyelid lacerations.
Be suspicious in glancing blunt trauma to the cheek or zygoma. This type of blow puts a great deal of stress on the medial canthal anatomy and may result in avulsion of the medial canthus with coexistent canaliculus laceration. This type of laceration may be missed because of the blunt mechanism and because the medial canthal tissues often reappose into a reasonable position, masking the extent of the injury. Look for displacement, excessive rounding, or abnormal laxity of the medial canthus.
Dog bites are notorious for causing canalicular lacerations. Canalicular probing should be performed in all such cases, even with lacerations that appear to be superficial. In some cases, debridement of necrotic tissue is warranted. With uncooperative children, conscious sedation or examination under anesthesia is often necessary to thoroughly examine the eyelids and globes. Administer systemic antibiotics if contamination or foreign body is suspected. For animal bites, consider rabies prophylaxis if warranted. Note that canalicular lacerations are not an ophthalmological emergency and repair can be delayed for 3-7 days without long term negative effects.
Visible orbital fat in an eyelid laceration indicates penetration of the orbital septum, and all such patients require CT imaging and documentation of levator and extraocular muscle function. Exploration of deeper tissue planes may be needed and ophthalmology consultation is warranted. Do not remove any foreign body prior to surgery if there is a possibility of globe penetration or extension into the orbit – this is best performed in a controlled OR environment. A multi-disciplinary approach may be necessary with ophthalmology and possibly ENT or neurosurgery depending on the extent of the injury.
When repairing lacerations, try to not overdo the subcutaneous lidocaine, because it can cause tissue distortion and make the repair more challenging, though you must find a balance to keep the patient comfortable during the repair. It is advisable to place a drop of proparacaine and a protective shell over the eye to prevent any inadvertent globe trauma. I prefer to use absorbable vicryl or gut sutures in children or if the patient seems unlikely to follow up. I enjoy your clever OPTIC mnemonic, and agree all of those listed scenarios should result in an ophthalmology consult.
Rehan M. Hussain, MD
Ophthalmology
Retina Associates, Ltd
How to Cite this Post
[Peer-Reviewed, Web Publication] Hajjar, M, Bode, J. (2020, May 4). Eyelid Lacerations [NUEM Blog. Expert Commentary by Hussain, R]. Retrieved from http://www.nuemblog.com/blog/eyelid-lac
Is Fracture Healing Impaired by NSAIDs?
Written by: Andra Farcas, MD (PGY-3) Edited by: Jessica Bode, MD (NUEM ‘19) Expert Commentary by: Matthew Levine, MD
Clinical Question:
Are we impeding our patients’ fracture healing by giving them NSAIDs?
Why is this important?
Broken bones hurt. A lot. And we want to do something about that to make our patients feel better. In the context of the current opioid crisis and the controversy of prescribing opioids, we need good non-opioid alternatives. Enter NSAIDs. Multiple studies have investigated whether nonsteroidal anti-inflammatory drugs (NSAIDs) are efficient pain relievers in multiple scenarios, including fractures, and the consensus seems to lean towards yes. However, this is not a discussion about their effectiveness but rather an attempt to finally answer the question that emergency docs seem to have different answers to: will giving patients NSAIDs for their fracture-related pain actually lead to worse outcomes in terms of fracture healing?
Mechanism of action
NSAIDs work by inhibiting the COX enzyme that catalyzes the conversion of arachidonic acid into prostaglandins. In turn, prostaglandins work as inflammatory response mediators. When a bone is fractured, the healing process involves an inflammatory response. Giving NSAIDs alters that inflammatory response by decreasing prostaglandin production. This is why it’s been proposed that giving NSAIDs to patients with bone fractures will affect their healing.
Additionally, prostaglandins also modify the expression of bone morphogenic proteins (BMPs), which are involved in the bone healing process. This is another mechanism by which NSAIDs may affect fracture healing.
What the Research Shows
There have been a multitude of animal and human studies investigating the effect of NSAIDs on bone fracture healing, mostly in the orthopedic surgery community. The chart below has short summaries of some of the ones that are most relevant to emergency medicine.
Studies with Animals
Animal studies in general tend to use mice or rats with induced long bone fractures. The animals are exposed to NSAIDs or placebo and various bone characteristics are measured at various time frames. Capello, 2013 and Utvag, 2010 showed that Ketorolac, Parecoxib, and Diclofenac had no effect on strength, stiffness, or bone mineralization in rats with tibia fractures. On the other hand, Lu, 2012 and Murnaghan, 2006 showed that Indomethacin was associated with decreased bone and cartilage formation and that Rofecoxib was associated with slower and poorer healing in mice with tibia and femoral fractures, respectively.
Studies with Humans
While studies with animals have a lot of advantages, we’re often more interested in clinical outcomes than physiological nuances. DePeter 2016 in a retrospective chart review showed no association between ibuprofen exposure in kids with various fractures and healing complications like nonunion, delayed union, or re-displacement. However, there was no specification of the timeframe of treatment, and some patients received ibuprofen in the ED while others were sent home with it without a clear defined use period. Adolphson, 1993 showed there was no significant difference in bone mineral decrease in postmenopausal women with displaced Colles’ fractures that used Piroxicam for 8 weeks after the fracture compared to women who used a placebo. Giannoudis, 2000 found greater proportion of NSAID use in patients with nonunion of a femur shaft fracture compared to those who had union and found that in patients who had union, those who used NSAIDs took longer to achieve it. Bhattacharyya 2005 found that NSAID use at 61-90 days post fracture was associated with nonunion. However, an important thing to point out is that association does not equal causation. Is it that the NSAID use caused the poor outcome? Or is it that the poor outcome was more painful and thus those patients used pain medication for longer. To that point, Bhattacharyya, 2005 also found that there was an association between opioid use at 61-90 days and nonunion.
The Conclusion
The evidence isn’t slam-dunk in either direction on whether using NSAIDs impedes the fracture healing process. There aren’t many randomized control trials to explore causation (versus association) of NSAID use with fracture healing outcome. The one RCT I could find (Adolphson, 1993) leans towards no difference in outcome between NSAID users and placebo users. My takeaway: if my patients have no other contraindications to using NSAIDs and if their pain is well-controlled with said medication, then I’m going to advise they can use it for a short term and advise them to seek medical attention if they’re still needing to use NSADs regularly a few weeks out.
Expert Commentary
As is frequently the case in medicine, confounding factors make seemingly simple questions have not-so-simple answers.
Some animal studies suggested that NSAIDS can do harm to healing fractures. Others did not. The benefit of the animal study approach is that more variables can be controlled - the site of fracture, NSAID type, dose, frequency, duration of therapy. These animals were also surely more compliant with medications and follow up than our patients. However, animal studies do not necessarily translate to human outcomes. And these animal studies were less than definitive anyway.
The human studies were also less than definitive. Human studies are difficult for many reasons. Do NSAIDS affect pediatric, adult, elderly bone healing outcomes the same? Does it matter which NSAID, how often it is taken, the dose, the duration of use, which bone is fractured? When taking all these factors into consideration, it becomes more clear just how unclear the answer to this question is.
So at the end of the day, we do what we do time and time again in medical decision making – a risk-benefit analysis:
Option 1: Give NSAIDS. Risk causing an uncertain NSAID-related complication such as poor bone healing or a known NSAID complication such as a cardiovascular/GI/renal issue. Avoid narcotics.
Option 2: Give acetaminophen. Little downside as long as the patient can follow the directions you give them or on the bottle to avoid overdosing. Avoid NSAIDS and narcotics.
Option 3: Give narcotics. Avoid complications of NSAIDS. Expose to complications of narcotics. I don’t need to list these.
Option 4: Some combination of options 1, 2, and 3 because your gestalt is telling you that acetaminophen or NSAIDS alone won’t cut it for some cases.
I have different patients that end up falling into each of those options. This also raises another question - how do NSAIDS, acetaminophen, and opiates compare to each other for control of fracture pain? These scenarios and questions again demonstrate that medicine is often not a robotic one-size-fits-all, one-answer-to-every-question field, or else Dr. Google would replace us. Until more definitive RCTs come along, you will be required to use your judgment, or as I like to call it, expertise.
Matthew Levine, MD
Associate Professor
Department of Emergency Medicine
Northwestern University
How to Cite This Post
[Peer-Reviewed, Web Publication] Farcas A, Bode, J. (2020, April 6). Clinical Question: are we impeding our patients’ fracture healing by giving them NSAIDs? [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/fx-nsaids
Other Posts You Might Enjoy…
References
1. Adolphson, P., Abbaszadegan, H., Jonsson, U., Dalen, N., Sjoberg, H.E., Kalen, S. No effects of piroxicam on osteopenia and recovery after Colles’ fracture: A randomized, double-blind, placebo-controlled prospective trial. Archives of Orthopaedic and Trauma Surgery, 1993; 112: 127-130.
2. Bhattacharyya, T., Levin, R., Vrahas, M.S., Solomon, D.H. Nonsteroidal Antiinflammatory Drugs and Nonunion of Humeral Shaft Fractures. Arthritis & Rheumatism (Arthritis Care & Research), 2005; 53(3): 364-367.
3. Cappello, T., Nuelle, J.A.V., Katsantonis, N., Nauer, R.K., Lauing, K.L., Jagodzinski, J.E., Callaci, J.J. Ketorolac Administration Does Not Delay Early Fracture Healing in a Juvenile Rat Model: A Pilot Study. Journal of Pediatric Orthopaedics, 2013; 33(4): 415-421.
4. DePeter, K.C., Blumberg, S.M., Becker, S.D., Meltzer, J.A. Does the use of ibuprofen in children with extremity fractures increase their risk for bone healing complications? The Journal of Emergency Medicine, 2017; 52(4): 426-432.
5. Dodwell, E.R., Latorre, J.G., Parsini, E., Zwettler, E., Chandra, D., Mulpuri, K., Snyder, B. NSAID Exposure and Risk of Nonunion: A Meta-Analysis of Case-Control and Cohort studies. Calcific Tissue International, 2010; 87: 193-202.
6. Giannoudis, P.V., MacDonald, D.A., Matthews, S.J., Smith, R.M., Furlong, A.J., De Boer, P. Nonunion of the femoral diaphysis: the influence of reaming and non-steroidal anti-inflammatory drugs. The Journal of Bone & Joint Surgery, 2000; 82-B(5): 655-658.
7. Lu, C., Xing, Z., Wang, X., Mao, J., Marcucio, R.S., Miclau, T. Anti-inflammatory treatment increases angiogenesis during early fracture healing. Artchives of Orthopaedic and Trauma Surgery, 2012; 132: 1205-1213.
8. Murnaghan, M., Li, G., Marsh, D.R. Nonsteroidal Anti-Inflammatory Drug-Induced Fracture Nonunion: An Inhibition of Angiogenesis? The Journal of Bone and Joint Surgery, 2006; 88-A(3): 140-147.
9. Utvag, S.E., Fuskevag, O.M., Shegarfi, H., Reikeras, O. Short-Term Treatment with COX-2 Inhibitors Does Not Impair Fracture Healing. Journal of Investigative Surgery, 2010; 23: 257-261.
10. Yates, J.E., Shah, S.H., Blackwell, J.C. Do NSAIDs impede fracture healing? The Journal of Family Practice, 2011; 60(1):41-42.
Non-Accidental Trauma - A Can’t Miss Diagnosis
Written by: Dana Loke, MD (NUEM PGY-4) Edited by: Ashley Amick, MD (NUEM ‘18) Expert commentary by: Lauren Riney, DO
Introduction
Non-accidental trauma (NAT) is a leading cause of pediatric traumatic injury and death. In 2014 alone, there were 1546 reported deaths from NAT and 3.6 million child abuse referrals submitted to Child Protective Services (CPS). [1] NAT is most commonly encountered in young children, but can occur at any age. The classic signs and symptoms of NAT will be reviewed here, but it is important to realize that occult injury is common. Compared with accidental pediatric trauma, patients with NAT have been shown to have higher injury severity scores, rates of intensive care unit admission, and mortality. Furthermore, the diagnosis of NAT is delayed in 20% of cases, increasing the risk of poor outcomes.[2] Therefore, the Emergency Physician (EP) must maintain a high index of suspicion for NAT to prevent the grave consequences of missed diagnosis for the patient and any other children in the home.
Red Flags and Risk Factors
NAT is a frequently missed diagnosis, but there are some red flags and risk factors that should make the EP take pause and consider this diagnosis. Children at greatest risk are generally toddler and younger, and often come from dysfunctional family units. A recent study found that 97% of NAT cases have antecedent familial dysfunction, such as substance abuse (alcohol or drugs), psychiatric disorder, history of violence or incarceration, or child withdrawal. [3] Additionally, over 70% of reported NAT deaths in 2014 were in children under 3 years old. [1]
Red Flags
Injuries inconsistent with the caregiver’s history
Reported mechanism of injury is unexpected for the child’s developmental status (for instance, a 2 week old infant rolling off of a bed)
Delayed presentation
Risk Factors
Age under 5 account for 81.5% of cases; children under 1 are most vulnerable [3]
Prematurity
Multiple medical conditions
Young parent
Female parent (although males are more likely to inflict fatal NAT)
Poor social support
Unplanned or unwanted pregnancy
Poor prenatal care
Shorter birth intervals between children
Increased number of separations from the child in the first year
Abuser Characteristics
Poor self-esteem
Depression and suicide attempts
Life stressors
Personal history of being abused as a child
Exposure to foster care or abandonment as a child
Engagement in criminal activity or corporal punishment as a child
Many other suspected risk factors have been studied. There is no consensus regarding whether a particular race is at greatest risk for NAT however black children have a greater risk of mortality from NAT. [4] Similarly, there is no consensus regarding socioeconomic status as it relates to NAT risk, but studies have shown that incidence of non-accidental head trauma and its severity rise during times of economic recession. [4]
Presentation
Figure 1: Bruising patterns that suggest child abuse. [6]
Bruising
Bruising is the most common manifestation of NAT but has low specificity. In any child presenting with bruising, it is imperative to note the location, shape and pattern of the lesion and ensure this is clearly documented. Bruising located over soft tissue areas such as the cheeks, neck, genitals, buttocks, torso, and back, are more likely to represent NAT than bruises over bony prominences. [4] The shape of the bruise should be considered as well, since the bruise often reflects the shape of the causative object. Common objects used to inflict injury include belts, cords, shoes, kitchen utensils, hangers, and teeth. [4] Additionally, patterned bruises should raise suspicion for NAT since they generally do not occur with accidental trauma. Lastly, any bruising in non-mobile infants is suspicious for NAT as well. [5]
Figure 2: Forced immersion burn of buttocks with bilateral, symmetric leg involvement in a “stocking” pattern. [7]
Burns
Burns occur in 8-12% of NAT cases. [2] The most common types of burns from NAT are scald burns and thermal contact burns. Scald burns are the most common and typically occur from forced immersion in hot liquids, usually of the buttock, or in a stocking-and-glove distribution. Scald burns generally have sharp demarcation, uniform depth, and lack splash or drip marks that would be seen in an accidental immersion. Thermal burns occur from contact with hot objects, of which branding with metal implements or cigarettes is a common presentation. Concerning features of burns include:
Location on the hands (especially the dorsum), legs, feet, or buttocks
Patterned contact burns in the shape of an object (such as a fork, clothing iron, curling iron, or cigarette lighter)
Sharp stocking-and-glove pattern with sparing of the flexed protected areas (the classic forced immersion burn pattern)
Figure 3: Classic metaphyseal lesion. White arrows denote femoral metaphyseal separation and black arrow denotes a proximal tibial lesion or “bucket handle.” [1]
Fractures
There are various non-accidental fracture patterns, several with high specificity as described below:
Classic metaphyseal lesion (CML) – Also known as “bucket handle fractures” or “corner fractures,” these fractures are highly specific in children less than one year old. They result from a shearing force applied to a long bone, which causes avulsion of the metaphysis. These fractures are not associated with falls.
Multiple posterior and/or lateral rib fractures – These fractures also have a high correlation with NAT in children less than one year old. They arise from a specific mechanism – grasping the child around the torso and exerting a squeezing/compressive force. These fractures are more likely to affect the rib head and neck given the closer proximity to the transverse processes of the spine. NAT should especially be considered when healing fractures are found in a child without recent CPR.
Figure 4: Posterior and lateral rib fractures of differing ages indicative of NAT [4]
Clavicular fractures and spiral fractures of long bones in nonambulatory children
Multiple fractures, especially if in different stages of healing
Scapular fractures
Sternal fractures
Spinous process fractures
Of note, spiral fractures of long bones generally result from twisting injuries (indicating NAT), but can occur accidentally from falls in ambulatory children. Therefore, these fractures (especially if coupled with clavicular fractures) are more specific for NAT in younger patients, and the specificity decreases with advancing age. Other described non-accidental patterns to consider include epiphyseal separations, vertebral body fractures and separations, digital fractures, linear and complex skull fractures, and subperiosteal bone formation. These patterns have low to moderate specificity for NAT. [1]
Abusive Head Trauma
Abusive head trauma (AHT) is the most fatal form of non-accidental injury in children. In fact, about 80% of deaths from NAT are caused by AHT and only 15% of patients with AHT survive without any sequelae. [4] AHT is a spectrum of injuries including collisions with stationary objects, direct blows to the head, and a repetitive acceleration- deceleration injury, also known as “Shaken Baby Syndrome.” Infants are particularly vulnerable to traumatic brain injury from shaking due to the relative weight of the head compared to the body, coupled with weak neck musculature. [1] If AHT is suspected, a non-contrast head CT should be obtained even with a nonfocal neurologic examination, because occult intracranial injury is common. Make sure to use age-appropriate dose reduction to minimize radiation exposure and if the CT scan is normal, consider further work-up with an MRI.
Figure 5: Fundus of child with AHT with too-numerous-to-count retinal hemorrhages indicated by the black arrows. [8] The white arrow indicates small pre-retinal hemorrhages. The white arrowhead denotes hemorrhage extending into the peripheral retina. The black arrowhead denotes a healthy optic disc.
Ocular Manifestations
Although there are many ocular manifestations associated with non-accidental head injuries, retinal hemorrhages occur most often (about 60-85% of non-accidental head injuries). [4] Suspicion for NAT should be especially heightened when retinal hemorrhages are found in combination with signs of head trauma. Other ocular manifestations of NAT include periorbital hematoma, eyelid laceration, subconjunctival hemorrhage, subluxed or dislocated lens, cataracts, glaucoma, anterior chamber angle regression, iridiodialysis, retinal dialysis or detachment, intraocular hemorrhage, optic atrophy, or papilledema. [4]
Management and Disposition
All patients with suspected NAT should be admitted for protection and coordination of care even if they are clinically stable. Child Protective Services (CPS) must be notified, and engagement with the institutional social worker and child abuse team is recommended. It is important to note patients with NAT often have worse outcomes than other assault patients despite similar mechanisms of injury with intent to harm. [9] These patients often require close monitoring with Intensive Care Unit (ICU) resources. Patients with NAT should undergo a full skeletal survey as indicated in Figure 6 with additional imaging (CT, MRI) tailored to each patient. For instance, CT abdomen and pelvis should be obtained per general trauma guidelines, particularly if there is suspicion for solid organ or visceral injury.
Figure 6: Elements of the Skeletal Survey. Although a full skeletal survey is currently the standard of care for patients with NAT, there are ongoing research efforts to tailor X-ray imaging more specifically to each patient. [1]
Other diagnoses to consider in these patients include metabolic bone disease (such as rickets, Caffey disease, and osteogenesis imperfecta), blood dyscrasias, benign enlarged subarachnoid spaces (BESS), glutaric aciduria type 1 (which causes brain atrophy and subdural fluid collections). [1] However NAT is far more common than these diagnoses and carries significant morbidity and mortality when overlooked so should be considered and worked-up prior to these diagnoses.
Key Points
Pediatric NAT causes significant morbidity and mortality, and therefore EPs must maintain a high degree of suspicion for this diagnosis.
Red flags during evaluation include a changing or inconsistent history, injuries inconsistent with the history, an unexpected mechanism of injury based on the child’s developmental status, and delayed presentation despite significant injury.
Risk factors for NAT include children younger than school age (with children younger than 1 being most vulnerable), family dysfunction, prematurity, multiple medical conditions, young/female parent, poor social support, unplanned or unwanted pregnancy, poor prenatal care, numerous separations from the child in the first year of life, and history of psychiatric issues, stressors, criminal activity, or childhood abuse or abandonment in the abuser.
Although physical exam findings can be non-existent or non-specific, highly specific findings include bruising over soft tissue areas; bruises/burns that are patterned take the form of an object; any bruising in a non-mobile child; scald burns on the hands, legs, feet, or buttocks; and stocking-and-glove patterned burns.
Highly concerning fracture patterns include classic metaphyseal lesions (“bucket handle fractures” or “corner fractures”), multiple posterior and/or lateral rib fractures, clavicular or spiral long bone fractures in any nonambulatory child, multiple fractures, fractures in different stages of healing, scapular fractures, sternal fractures, and spinous process fractures.
There is a wide range of ocular manifestations in NAT but the most common manifestation is retinal hemorrhage(s).
AHT carries the highest mortality rate of all the injuries associated with NAT. Any suspicion for AHT warrants consideration of a non-contrast head CT.
Notify Child Protective Services (CPS) and admit these children for further NAT work-up including a full skeletal survey.
Expert Commentary
Excellent overview of NAT in the Emergency Department with emphasis on risk factors and manifestations. I want to add a few pearls about NAT and then will focus my commentary on NAT management in the ED as well as discussion with families, as this was recently a large quality improvement project in our pediatric tertiary care center.
Neglect is the most common form of child abuse accounting for about two-thirds of all forms of abuse and often accompanies other forms of abuse. (1) Neglect is involved in about 50% of all cases of fatal child abuse. (1) Among children less than 1 year of age, 25% of fractures are a result of abuse. (2) Consider two things: does the explanation the provider stated account for the fracture the child has sustained? Is the child developmentally capable of the action being described? After 2 years of age, the history and physical exam should determine the imaging required. Over 5 years of age, the yield of unsuspected fractures from a skeletal survey is only 9%, making this group more amenable to selective radiographic studies. (3)
Diagnosis of NAT in children remains a challenge due to provider bias, preconceptions, and failure to recognize the presentation as possible abuse. (4,5) As a result, these injuries may go undetected, leading to further injury prior to diagnosis. An estimated 25% of children ultimately diagnosed with NAT have a sentinel injury prior to their abuse diagnosis. (6,7) Of abused children with a previous sentinel injury, the most common were a bruise (80%), a torn frenulum (11%), or a fracture (7%). (8) A large retrospective chart review estimated 80% of deaths from unrecognized abusive head trauma may have been prevented by earlier detection of NAT. (6) The American Academy of Pediatrics (AAP) states that “ANY injury to a young, pre-ambulatory infant” suggests abuse. (9)
Figure 1: Standardized Physical Abuse Guideline.
At our institution, a team of pediatric emergency medicine physicians and child abuse pediatricians convened to develop and implement a standardized NAT guideline for providers in the ED when evaluating children with suspected NAT (Figure 1 Standardized Physical Abuse Guideline). This work stemmed from a chart review showing there was significant variability in the evaluation and management of children with concern for NAT in our Pediatric Emergency Department. The guideline was based on current peer reviewed literature as well as local expert consensus. It is divided into three separate age groups: < 6 months, 6-12 months, and >12-36 months. Age groups were determined based on risk of injury at different age levels in described literature, acquisition of milestones as age progresses, and increased ability for young children to show specific signs of injury with increasing age.
Lastly, the evaluation of NAT is stressful for both families and healthcare providers. The second page of our NAT guideline gives a sample script for EPs when discussing the non-accidental trauma evaluation for children. It states, “Any time a child comes to the hospital with this injury/these injuries, we evaluate for other injuries. Sometimes a child can have internal injuries such as fractures, head injury or abdominal injuries that we cannot see on the outside. Just like you, we want to make sure that your child is okay, so it is important to do this testing. We will also have our social worker come talk to you. This is a standard part of our evaluation. We are happy to answer any questions along the way”. It is important to acknowledge that this process is stressful, time consuming, and not comfortable for the child. Explaining each part of the process is important. Ensure that you use language that is non-accusatory. As EPs, we are not the ones to identify who the perpetrator is/was, but rather ensure the full NAT evaluation is completed and allow social work and/or Child Protective Services to determine further action.
Non-accidental trauma remains too prevalent in our country. Literature continues to show that unrecognized NAT leads to worse injuries and sometimes fatality. Continuing knowledge and education about injuries suspicious for NAT for EPs remains imperative. Standardized evaluations and real time order sets can increase appropriate management of NAT in the Emergency Department.
References:
Dubowitz H. Epidemiology of Child Neglect. CAN 2011, pp 28-34.
Kaczor K, Clyde Pierce M. Abusive Fractures. CAN 2011, pp 275-295.
Martich KV. Imaging of Skeletal Trauma in Abused Children. CAN 2011, pp 296-308.
Higginbotham N, Lawson KA, Gettig K, et al. Utility of a child abuse screening guideline in an urban pediatric emergency department. J Trauma Acute Care Surg. 2014;76(3):871-877.
Tiyyagura GK, Gawel M, Koziel JR, et al. Barriers and facilitators to detecting child abuse and neglect in general emergency departments. Annals of Emergency Medicine. 2015;66(5):447-454.
Jenny C, Hymel K, Ritzen A, et al. Analysis of missed cases of abusive cases of head trauma. JAMA. 1999;282:621-6.
Rangel EL, Cook BS, Bennett BL, et al. Eliminating disparity in evaluation for abuse in infants with head injury: use of a screening guideline. Journal of Pediatric Surgery. 2009; 44(6):1229-34.
Sheets LK, et al. Injuries in Infants Evaluated for Child Physical Abuse. Pediatrics. 2013, pp 701-707.
Christian CW, Committee on Child Abuse and Neglect. The evaluation of suspected child physical abuse. Pediatrics. 2015;135:e1337–e1354.
Lauren C. Riney, DO
Assistant Professor
Division of Emergency Medicine
UC Department of Pediatrics
How to Cite this Post
[Peer-Reviewed, Web Publication] Loke D, Amick A. (2019, Oct 7). Non-Accidental Trauma. [NUEM Blog. Expert Commentary by Riney C]. Retrieved from http://www.nuemblog.com/blog/nonaccidental-trauma.
Other Posts You Might Enjoy
References
Pfeifer, C.M., Hammer, M.R., Mangona, K.L., & Booth, T.N. (2017). Non-accidental trauma: the role of radiology. Emerg Radiol, 24, 207-213.
Kim, P.T. & Falcone, R.A. (2017). Non-accidental trauma in pediatric surgery. Surgical Clinics of North America, 97.1, 21-33.
Child maltreatment 2014. Report, Children’s Bureau. Washington, DC: U.S. Department of Health and Human Services; 2014. Available at: http://www.acf. hhs.gov/sites/default/files/cb/cm2014
Paul, A.R. & Adamo, M.A. (2014). Non-accidental trauma in pediatric patients: a review of epidemiology, pathophysiology, diagnosis and treatment. Transl Pediatr, 3, 195-207.
Maguire, S., Mann, M.K., Sibert, J. & Kemp, A. (2005). Are there patterns of bruising in childhood which are diagnostic or suggestive of abuse? A systematic review. Arch Dis Child, 90, 182-186.
Boos, S.C. (2017). Physical child abuse: Recognition. Retrieved April 21, 2017, from http://www.uptodate.com
Hobbs, C.J. (1986). When are burns not accidental? Archives of Disease in Childhood, 61, 357-361.
Binenbaum G., Rogers, D.L., Forbes, B.J., Levin, A.V., Clark, S.A., Christian C.W., Liu, G.T., & Avery R. (2013). Patterns of retinal hemorrhage associated with increased intracranial pressure in children. Pediatrics, 132, 430-434.
Litz, C.N., Ciesla, D.J., Danielson, P.D. & Chandler, N.M. (2017). A closer look at non-accidental trauma: Caregiver assault compared to non-caregiver assault. Journal of Pediatric Surgery, 52, 625-627.
Pelvic Fractures
Written by: Justine Ko, MD (NUEM PGY-3) Edited by: Terese Whipple, MD (NUEM PGY-4) Expert commentary by: Matt Levine, MD
Pelvic Ring Fractures
Overview
Pelvic ring fractures make up about 3% of skeletal fractures [1]. In the emergency department, they are often seen with high-impact blunt trauma, such as MVCs, crush injuries, or falls.
The pelvis consists of the sacrum and the two innominate bones, which are made up of the ilium, ischium, and pubis [2]. The sacrum and the innominate bones together form a posterior arch and an anterior arch that join to make a ring, also known as the pelvic inlet. The stability of the pelvis is enforced by ligaments: the sacroiliac ligaments, the sacrospinous ligaments, and sacrotuberous ligaments [2]. The ligaments and bones together give the pelvis vertical and rotational stability.
Pelvic ring fractures carry a significant mortality, ranging from 15-25% in closed fractures, and as much as 50% in open fractures [3]. They are often associated with other high-mortality injuries as well, such as chest injuries, spinal injuries.
What are the mechanisms associated with these injuries?
The Young-Burgess classification system categorizes injury patterns based on the mechanism of injury and the forces applied: anteroposterior compression (APC), lateral compression (LC), and vertical shear (VS) [1]. The most common mechanism is lateral compression, which occurs when lateral forces are applied to the pelvis (as the name would suggest). APC injuries result in the classic “open book” pelvic fractures (Figure 1).
Figure 1
Figure 2
Anteroposterior compression: causes disruption of the pubic symphysis. A normal pubic symphysis should not exceed 5mm. Injuries where the symphysis widening is <2.5cm, with no significant posterior column injury or other fractures, are usually considered stable. APC injuries are often associated with significant blood loss, as the pelvic volume increases and can store more blood [4].
Lateral Compression: considered rotationally unstable. This mechanism causes the pelvic volume to decrease, and therefore, is associated with less blood loss [4].
Vertical shear: the most unstable, often associated with axial loading of the hemipelvis, such as with a fall or a jump from a height.
What exam findings should I look for?
During a trauma workup, visual inspection for leg-length discrepancy or rotation of the pelvis may provide clues to a hip or pelvic injury. You may assess for stability of the pelvis by applying downward and medial pressure to the bilateral iliac crests. Apply pressure gently and slowly to avoid disruption of any possible clots that may have formed.
A digital rectal exam should also be performed to assess for spinal cord injury and frank blood. In males, the meatus should be assessed for possible bladder or urethral injury.
X-ray reading tips
Check the 3 circles! (Figure 3)
Pubic inlet
Two obturator foramina
Check for irregularities along the circles
In general, a vertical fracture pattern will be associated with APC or VS mechanisms. Horizontal fracture patterns will be associated with LC. [7]
Figure 3
Check the lines!
ilioischial line (orange line in Figure 4)- disruption suggest posterior column fracture [5]. The posterior column involves the posterior half of the acetabulum and extends to the ischial tuberosity [4].
iliopectineal line (red line in Figure 4)- disruption suggests anterior column fracture [5]. The anterior column involves the anterior half of the acetabulum and extends to the lateral half of the superior pubic ramus [4].
It’s a circle! If it breaks in one location, it likely broke in another place. Be wary of isolated rami fractures in the setting of high-impact trauma!
Figure 4
Management
As always, still stick to your ABCs!
Initiate blood transfusion early for patients in shock
Hemodynamic instability occurs in about 10% of these injuries [1]
Try to obtain IV access above the pelvis if a pelvic fracture is suspected.
Stabilize the pelvis with a TPOD or a sheet for patients with open book pelvic fractures
Apply over the GREATER TROCHANTERS
Do not apply a compressive device for isolated lateral compression pelvic fractures as the device can cause further compression and worsen the displacement [4].
If the hemorrhage or hypotension is suspected to be from pelvic hemorrhage, activate IR early.
If stable, obtain a CTA of the pelvis to assess for vascular injury
What other injuries should I look for?
Urologic Injury: incidence in pelvic fractures is ~5%. Patient with blood at the meatus will need a retrograde urethrogram and then a cystogram [4].
Neurologic Injury: pelvic fractures can be associated with plexus injuries and even cauda equina syndrome. Check for rectal tone and saddle anesthesia [4].
Gynecologic injury: blood a the introitus can represent urethral injury or an open pelvic fracture [4].
Expert Commentary
Dr Ko has provided a high yield concise guide of the key cognitive and clinical features of pelvic fractures. A few points worthy of further emphasis:
When examining for pelvic instability, do not rock on the pelvis to further open it. Instead, compress medially to feel instability while closing, rather than opening, the pelvis.
For most studies we order, we have the luxury of waiting for a formal report. Pelvis x rays are one of the few radiographic studies that the Emergency Physician must be able to rapidly interpret independently at the bedside for patient management! Do not just eyeball the film. It is impossible to find multiple pelvic fractures that way. Go over every arc, curve, and ring visually. If something appears possibly abnormal, compare to the other side for (a)symmetry. And if you find one break in the pelvic ring, scrutinize for more, there almost always are. Many of these are in the posterior ring, the part of the pelvis for which plain films are least sensitive. CT will help evaluate the posterior elements if feasible.
Understanding the classic LC, APC, VS mechanism classifications lend insight into when a binder will help. Realize, however, that in vehicular trauma, often the forces are multidirectional, so there will be mixed injury patterns. Ask yourself what you are trying to achieve by placing the binder. If it is closing the open book to tamponade hemorrhage, then go for it! If it is to provide some stability to a VS injury so you can move the patient without their pelvis flopping around than OK but it won't be therapeutic. If it is an LC injury where the femoral head has crushed through the acetabulum, avoid using a binder, you will only further intrude bony fragments into the pelvis!
Call IR the instant you realize there is a bleeding pelvic fracture. It takes time to prepare the suite, especially overnight!
Matt Levine, MD
Assistant Professor of Emergency Medicine
Northwestern Feinberg School of Medicine
How To Cite This Post
[Peer-Reviewed, Web Publication] Ko J, Whipple T. (2019, July 22). Pelvic Fractures. [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/pelvic-fracture.
Other Posts You May Enjoy
References
Halawi MJ. Pelvic ring injuries: Emergency assessment and management. Journal of Clinical Orthopaedics and Trauma. 2015;6(4):252-258.
Khurana B, Sheehan SE, Sodickson AD, Weaver MJ. Pelvic ring fractures: What the orthopedic surgeon wants to know. RadioGraphics. 2014;34:1317-1333
Weatherford B. Pelvic ring fractures. Orthobullets website. https://www.orthobullets.com/trauma/1030/pelvic-ring-fractures. Accessed October 14, 2018.
Walls RM, Hockberger RS, Gauche-Hill M. (2018). Rosen's emergency medicine: Concepts and clinical practice (9th ed.). Philadelphia, PA: Elsevier/Saunders.
Murphy A, Jones J. Pelvic radiograph (an approach). Radiopaedia website. https://radiopaedia.org/articles/pelvic-radiograph-an-approach. Updated August 2018. Accessed October 14, 2018.
Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br Vol. 1988;70(1):1–12.
Singh, AP. Pelvic fractures: Presentation and treatment. https://boneandspine.com/pelvic-fractures-presentation-and-treatment/
Images:
Case courtesy of Dr Jeremy Jones, https://radiopaedia.org Radiopaedia.org. From the case https://radiopaedia.org/cases/28928" rID: 28928
Unstable Cervical Spine Fractures
Written by: Sarah Sanders, MD (NUEM PGY-4) Edited by: Alison Marshall, MD (NUEM Alum ‘17) Expert commentary by: Steve Hodges, MD
Fractures of the cervical spine are injuries that must be approached with caution. Some are stable, some are unstable, and mismanagement can lead to life-altering sequelae. Remembering which fractures fit into which category is imperative for optimum emergency department care.
A quick review of cervical spine anatomy is a helpful starting point:
All the cervical spine anatomy images are credited to Netter, FH Atlas of Human Anatomy, Sixth Edition.
All the cervical spine anatomy diagrams are credited to Agur, AMR & Dalley, AF of Grant’s Atlas of Anatomy: Twelfth Edition.
The common mnemonic “Jefferson Bit Off A Hangman’s Thumb,” is used to remember the unstable fractures, which will be reviewed in this post.
Ultimately, understanding the mechanism of injury is crucial in identifying and accurately managing these injuries. The below PV cards are organized by mechanism and tailored down to be an on-shift reference.
In conclusion, cervical spine injuries required a high index of suspicion and caution by the emergency medicine physician as their variability and potential for neurological impairment is high. Hopefully this review can provide you with additional insight and ease of memory when the next Level 1 trauma rolls through the door.
Expert Commentary
Thanks for this insightful post. You've done a really nice job in laying out the most salient points. It is important to have an understanding of the anatomy and this is a great review. Knowing the factors that put people a high risk for injury is also paramount. Certain chronic disease states and anatomic variances, as you note, do put select patient populations at risk for specific injury. The mechanism of injury is something we always talk about, but when it comes to neck trauma we need to really pay attention to all available history including paramedic reports, or even cell phone video, to get the best possible picture or the mechanism of injury. I can not stress enough the importance of a detailed neurological exam including sensation(s) and reflexes. Any asymmetric finding should raise your level of suspicion for severe injury. Moving to advanced imaging is especially important if there is a complaint of a focal neurological deficit; be that transient, subjective or blatantly obvious.
Athletes or motorcyclist who have suspected cervical spine injury, who have protective shoulder pads and/or helmets pose a unique challenge. Eventually the protective devices are going to need to be removed. There are many opinions on how best to do this and whether an x-ray should be done before attempting the removal of protective gear. From personal experience; it can be difficult to remove protective gear; I recommend getting "all hands on deck" and using an methodical slow organized approach. More recent thought is that cervical spine imaging should incorporate procedures for removal of equipment before initial radiographic evaluation.[1] Once the gear is removed a c-collar should then be applied and you can proceed with imaging.
Recommendations for imaging the cervical spine for trauma has changed quit a lot over the last several years. The National Emergency X-Radiography Utilization Study (NEXUS) and the Canadian C-Spine Rule (CCR) have been validated and have allowed our practice to advance such that we can effectively practice clinical medicine. However, a word of caution on using these criteria with patients who could be impaired. Sometimes the mild dementia, delirium or subtle drug, alcohol intoxication can lead us astray when we rely solely on these criteria. The cross table lateral films and specifically flexion/extension views have fallen out of favor. Most patients without focal neurological complaint or deficit are imaged with plain CT. If your patient has a focal neurological complaint or deficit, a suspected ligamentous or disc injury an MRI should be done. Depending on the exam and risk factors I would consider either a CTA or and MRA to evaluate for vascular injury.
You asked about c-collars specifically. What is available to you will be somewhat hospital - vendor specific. I prefer the Aspen or the Miami collar, they are very similar in function overall and superior to the pre-hospital EMS ones. When a patient has to be transported to another facility; make sure that the patient has full immobilization with back board and head-side blocks with the collar and head secured to the side blocks. An immobilized patient that requires intubation can make an easy air way difficult and a difficult airway terrifying. Make sure you have all your equipment prepared, including a surgical method, before you intubate. The person holding c spine immobilization needs to knows their role.....don't let go and don't move. This is the time when a video assisted intubation should be used. Use either the intubating bronchoscope or a video laryngoscope. This article talks a bit about managing airway in cervical spine injury and is a nice reference.[2]
Closing thoughts: maintain a high level of suspicion for injury in the setting of a focal neurological deficit, immobilize early immobilize often and don't be shy about intubating before transferring.
https://doi.org/10.1067/mem.2001.116333 Annals of Emergency Medicine; Baldwin et. al., "Football protective gear and cervical spine imaging" July 2001 Volume 38, Issue 1, Pages 26–30
10.4103/2229-5151.128013 International Journal of Critical Illness and Injury Science; Austin et. al., "Airway management in cervical spine injury" Jan-Mar; 4(1): 50–56
Steven W. Hodges, MD, FACEP
Assistant Medical Director
Northwestern Lake Forest Hospital
How To Cite This Post
[Peer-Reviewed, Web Publication] Sanders S, Marshall A (2019, January 21). Unstable Cervical Spine Fractures [NUEM Blog. Expert Commentary by Hodges S]. Retrieved from http://www.nuemblog.com/blog/cervical-spine-fractures.
Other Posts You May Enjoy
References
Adams, J. Lin, M. Mahadevan, SV. “Spine Trauma and Spinal Cord Injury.” Section VIII, Chapter 75. Emergency Medicine: Clinical Essentials. Second Edition. P652 - 657.
Agur, AMR; Dalley AF. Grant’s Atlas of Anatomy. Twelfth Edition. Chapter 4: Back. 2009.
Bergenheim, AT, Forssell, A. “Vertical Odontoid Fracture. Case Report.” Journal of Neurosurgery. Vol 74 (4) p665-667. 1991.
Netter, F. Atlas of Human Anatomy. Section Head & Neck. Sixth Edition. 2014.
Wheeless, CR. “Cervical Spine.” Wheeless Textbook of Orthopedics by Duke University. April 26 2016. 2 Jan 2017. http://www.wheelessonline.com/ortho/cervical_spine
REBOA
Written by: Andew Cunningham, MD (NUEM PGY-4) Edited by: Bill Burns, MD (NUEM Alum ‘17) Expert commentary by: Zaffer Qasim, MBBS, FRCEM, FRCPC(EM), EDIC
REBOA: Ready for Prime Time?
For years, the resuscitative thoracotomy has been the sole weapon in the physician’s arsenal against a loss of a perfusing pressure in the crashing trauma patient. With the advent of new endovascular technologies, novel methods to control hemorrhage are being refined, among them Resuscitative Endovascular Balloon Occlusion of the Aorta or REBOA. With this newer method getting a lot of attention in the emergency and trauma communities, it’s time to take a look at what it is, how successful it is, and where we are going with it.
What Is It?
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a possible alternative to resuscitative thoracotomy in cases of non-compressible torso hemorrhage (NCTH) that present to the Emergency Department in extremis.1
REBOA works via the insertion of a catheter through the femoral artery to allow an endovascular balloon to be deployed within the aorta, allowing for bleeding control and augmentation of afterload in hemorrhagic shock.2
REBOA can be deployed in Zone III of the Aorta, as depicted in the image below, for pelvic hemorrhage, or in Zone I of the Aorta for abdominal hemorrhage.3
Borrowed from Reference 3
The primary indications for REBOA include:
PEA arrest secondary to abdominal or pelvic hemorrhage within 10 minutes of the onset of arrest
Severe hypovolemic shock secondary to abdominal or pelvic hemorrhage
Unstable hemodynamics refractory to volume resuscitation in patients with abdominal or pelvic hemorrhage2
The major contraindications include age older than 70, significant comorbidities, prolonged PEA arrest (lasting longer than 10 minutes), or high suspicion for proximal aortic injury (REBOA may exacerbate bleeding from thoracic sources).2
Does It Work?
A study at U of Arizona showed that 45% of patients who received thoracotomy may have benefited from REBOA based on autopsy results, but only 32% of the patients receiving a thoracotomy did not have a contraindication for it, and of those who did not have a contraindication, only roughly half would have potentially benefited. Compared to prior literature, this may suggest that REBOA is not as useful in patients in extremis.1
Although the literature does suggest that REBOA reduces the amount of overall hemorrhage, there is still no definitive evidence in humans of a decrease in mortality.4
There are still risks of complications in humans, including arterial injury and limb ischemia.3 In animal models, REBOA has also resulted in renal failure, liver failure, intestinal ischemia, and multiple other injuries which result from occlusion of the aorta.5
Given that REBOA still obstructs distal flow, just like cross-clamping the aorta in a resuscitative thoracotomy, it is still reserved as a last resort maneuver. The effects of aortic occlusion can be reviewed below6:
Borrowed from Reference 6
Where Is It Going?
An alternative to REBOA may be Selective Aortic Arch Perfusion (SAAP); in this similar yet separate endovascular approach, a catheter that has two ports is utilized instead of the single-port REBOA catheter. This allows for both occlusion of the aorta and selective administration of blood, pressors, or other medications directly to the heart and brain. Where REBOA may be useful for exclusively shock, SAAP may have advantages in cardiac arrest secondary to trauma.7
A new, smaller REBOA catheter, the 7 French ER-REBOA, may cause fewer injuries and also allows for simultaneous blood pressure monitoring.8
Partial REBOA, or P-REBOA, allows for controlled blood flow to the body distal to the area of occlusion, in efforts to limit ischemia.5
Is It Feasible for ER Docs to Perform?
Yes! Some of the larger studies performed in Japan required placement by either a surgical or emergency medicine-trained attending.9
In England, there are case of REBOA being deployed in the pre-hospital setting to act as a bridging method for resuscitation during transport.7 As the relay between the hospital and Emergency Medical Services, it is an EM physician’s responsibility to be aware of this method and its utility in her area.
Take-Home Points
REBOA is a newer up-and-coming method of controlling hemorrhage secondary to abdominopelvic trauma that may act as an alternative to resuscitative thoracotomy.
Although more data still needs to be collected, REBOA has not yet shown to clearly improve mortality, and does come with certain risks and complications.
There are more novel methods of REBOA undergoing research and development, including SAAP, ER-REBOA, and P-REBOA, which may strengthen the utility of REBOA and reduce some of the complication risks.
REBOA is within an EM physician’s scope of practice, and may play a role in EMS in the future. As such, it is our duty to be aware of it and follow along with its developments.
Expert Commentary
Thanks for a great post on an evolving temporary hemorrhage control concept. Hemorrhage, and torso hemorrhage in particular, remains the largest cause of death in trauma in the first 24 hours. In the right patient, REBOA can be another effective procedure in the emergency physician’s toolbox. Some additional points to consider:
1. Access is key! The rate limiting step is early common femoral artery (CFA) access. It’s important to emphasize accessing the common as placing the sheath in one of the smaller branch vessels could increase the risk of iatrogenic injury. I advocate using ultrasound to define the anatomy and routinely placing a CFA arterial line in your “big sick” patients to maintain skills. As you state, this step is well within the wheelhouse of the Emergency Physician, and the foundation to build on to train in placing REBOA
2. Patient selection is critical! The available data generally has the inclusion/exclusion criteria listed, but definitions on who is “unstable” vary. In my opinion, an arbitrary blood pressure cutoff of <90mmHg in someone with torso hemorrhage should not automatically trigger REBOA. I think these patients should get a CFA line, and then proceed to REBOA only if not responding to initial resuscitative measures or rapidly deteriorating to imminent arrest.
3. Placement before arrest will likely lead to better outcomes. The evolving data shows that the group that benefits most in terms of mortality are the nonresponders who will imminently arrest unless they have a lifesaving procedure. In the arrested patient, as mentioned, determining the time of arrest is crucial. This can certainly be challenging with prehospital arrest.
4. While the data does not show improved mortality compared to thoracotomy, there does seem to be a trend to improved neurologically intact survival – this is our ultimate goal and speaks to the ability to use REBOA proactively, before traumatic arrest happens
5. It is absolutely critical that REBOA is used in a system that can rapidly deliver these patients to definitive care (OR/IR). The consequences of prolonged balloon occlusion as listed are dire. Based on collective clinical experience and translational animal data, I would not recommend occluding beyond 45 minutes to 1 hour in Zone 1.
6. REBOA in the US is currently used only at level 1 and 2 trauma centers. I think (as the British have shown), the biggest benefit is likely at smaller centers and ultimately prehospital. Success here will be based on procedural considerations (like p-REBOA to prolong safe inflation times), appropriate training, and systems issues (expedited transfer to definitive care).
Zaffer A. Qasim, MBBS, FRCEM, FRCPC(EM), EDIC
Assistant Professor of Clinical Emergency Medicine
UPenn Medicine
How to Cite This Post
[Peer-Reviewed, Web Publication] Cunningham A, Burns W (2019, January 7). REBOA [NUEM Blog. Expert Commentary by Qasim Z]. Retrieved from http://www.nuemblog.com/blog/REBOA
Other Posts You May Enjoy
References
Joseph B, Ibraheem K, Haider AA, et al. Identifying potential utility of resuscitative endovascular balloon occlusion of the aorta: An autopsy study. J Trauma Acute Care Surg. 2016;81:S128-S132.
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Vol 2016. LIFTL.
Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin. 2017;33:55-70.
Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80:324-334.
Perkins ZB, Lendrum RA, Brohi K. Resuscitative endovascular balloon occlusion of the aorta: promise, practice, and progress? Curr Opin Crit Care. 2016;22:563-571.
Russo RM, Neff LP, Johnson MA, Williams TK. Emerging Endovascular Therapies for Non-Compressible Torso Hemorrhage. Shock. 2016;46:12-19.
Bebarta V. REBOA - Ready for Prime Time? ACEP EM Education.
Weingart S. Podcast 170 - the ER REBOA Catheter with Joe DuBose. Vol 2016. EMCrit Blog.:Available at [http://emcrit.org/podcasts/er-reboa].
Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78:897-903; discussion 904.
Topical Hemostatics
Written by: Alex Ireland, MD (NUEM PGY-3) Edited by: Andrew Moore, MD (NUEM Alum ‘18) Expert commentary by: Joseph Posluszny, MD
Expert Commentary
The above summary of the mechanism of actions, indications for and limitations of topical hemostatic agents is comprehensive and thorough.
As with most summaries of hemostatic agents, we focus on the mechanism of action or aspect of coagulation by which the agent works. Clinically, it may be easier to take a different approach.
Sometimes, the most difficult aspect of applying a topical hemostatic agent is determining the appropriate agent given the clinical scenario- all work in some fashion, but which will work best?
To help approach this, an initial question to help frame what to do in the trauma bay or ED would be: does this wound require just a hemostatic agent as a covering/dressing to promote hemostasis or are both assisted hemostasis and pressure needed to control the bleeding? For superficial, low volume, but persistently bleeding wounds, topical agents like Dermabond, thrombin (with gelfoam) and Surgicel are ideal. For deep, complex wounds that require hemostatic agents and pressure, QuikClot Gauze, Ativene and Combat Guaze are more effective.
Hospitals and EMS systems purchase a variety of topical hemostatic agents. It is imperative to become familiar with these agents and be prepared for their indications before you are presented with bleeding uncontrolled by conventional dressings or pressure.
Joseph Posluszny, MD
Assistant Professor of Surgery
Trauma and Critical Care, McGaw Medical Center of Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Ireland A, Moore A (2018, December 10). Topical Hemostatics [NUEM Blog. Expert Commentary by Posluszny J]. Retrieved from http://www.nuemblog.com/blog/topical-hemostatics
Bad Blood
Written by: Ade Akhetuamhen, MD (NUEM PGY-2) Edited by: Spenser Lang, MD (NUEM Alum ‘18) Expert commentary by: Matthew Levine, MD
Expert Commentary
Dr Akhetuamhen has provided a nice quick reference for topical hemostatic agents (THAs). These agents have become more relevant in recent years, particularly in prehospital care, as the prehospital emphasis has shifted from resuscitating hemorrhage more towards hemorrhage control. Much of our knowledge of these dressings come from battlefield studies of major hemorrhage. Their use has been formally endorsed by the American College of Surgeons Committee on Trauma in 2014, particularly for junctional site hemorrhaging. Dr Akhetuamhen has listed the properties of the ideal THA. No current product fulfills all of these criteria.
Much of what we know about these agents comes from military studies. There are limitations to these studies. There are fewer human studies, and these tend to be retrospective, observational, and based on questionnaires. The possibility of reporting bias exists in these studies and study design made it impossible to control for the type of wound. There are far more animal studies. Animal studies allow for the ability to control for wound type, but are more difficult to simulate real life wounds from missiles or shrapnel.
Hemcon and Quickclot products were the earliest products studied by the military and became the early THA gold standards after they were determined to be more effective than standard gauze. An earlier concern for Quickclot was exothermic reactions from the activated products that caused burns to patients. As Quickclot transitioned its active ingredient from zeolite to kaolin, this concern diminished. Quickclot is available in a roll called Combat Gauze that is favored by the military and available in our trauma bay.
Finally, there are some important practical tips for using these products. THAs are not a substitute for proper wound packing and direct pressure. Most topical hemostatic agent failures in studies were from user failure! THAs must come into contact with the bleeding vessel to work. Simply applying the THA over the bleeding areas does not mean it is contacting the bleeding vessel. The product may need to be trimmed, packed, shaped or molded in order to achieve this. Otherwise it is simply collecting blood. After it is properly applied, pile gauze on top of it and give firm direct pressure for several minutes before checking for effectiveness.
See what THA(s) you have available in your trauma bay, it is nice to know ahead of time before presented with a hemorrhaging patient what you have and in what form (a roll, sponge, wafer, etc). Find out how the product is removed. It may not be relevant to the patient’s ED stay but at some point the dressing needs to come off. Some are left to fall off on their own. The chitosan products are removed by soaking them. When soaked, the chitosan turns slimy and can be slid off atraumatically.
Matthew Levine, MD
Assistant Professor of Emergency Medicine
Northwestern Medicine
How to Cite this Blog
[Peer-Reviewed, Web Publication] Akhetuamhen A, Lang S (2018, October 29). Bad Blood. [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/bad-blood



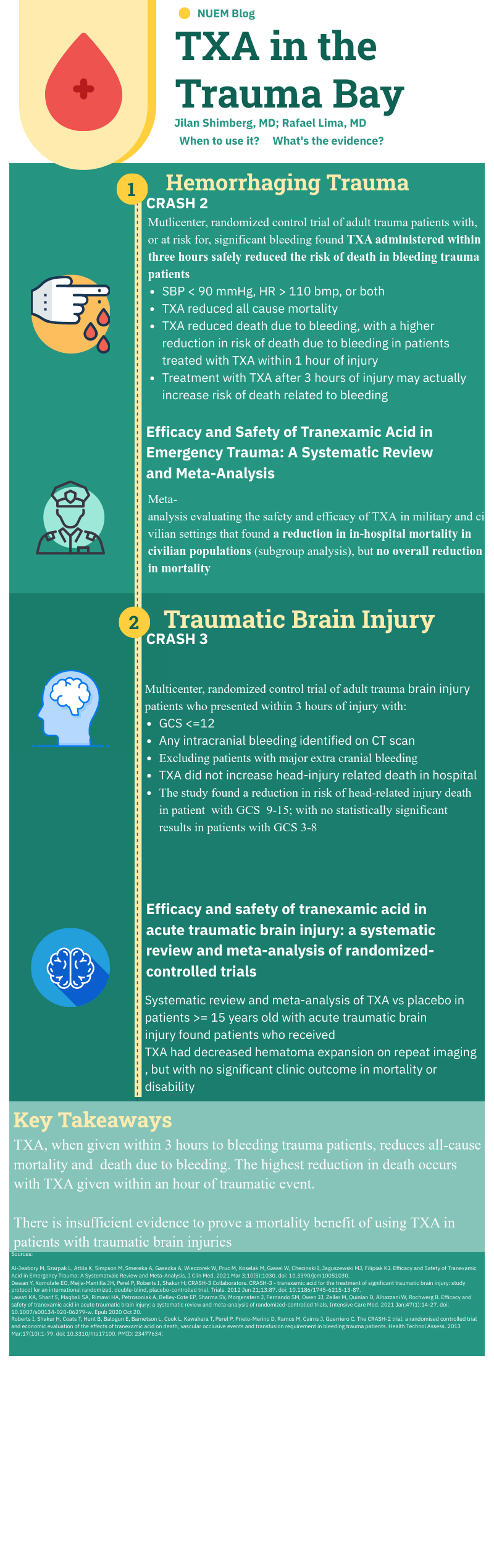



















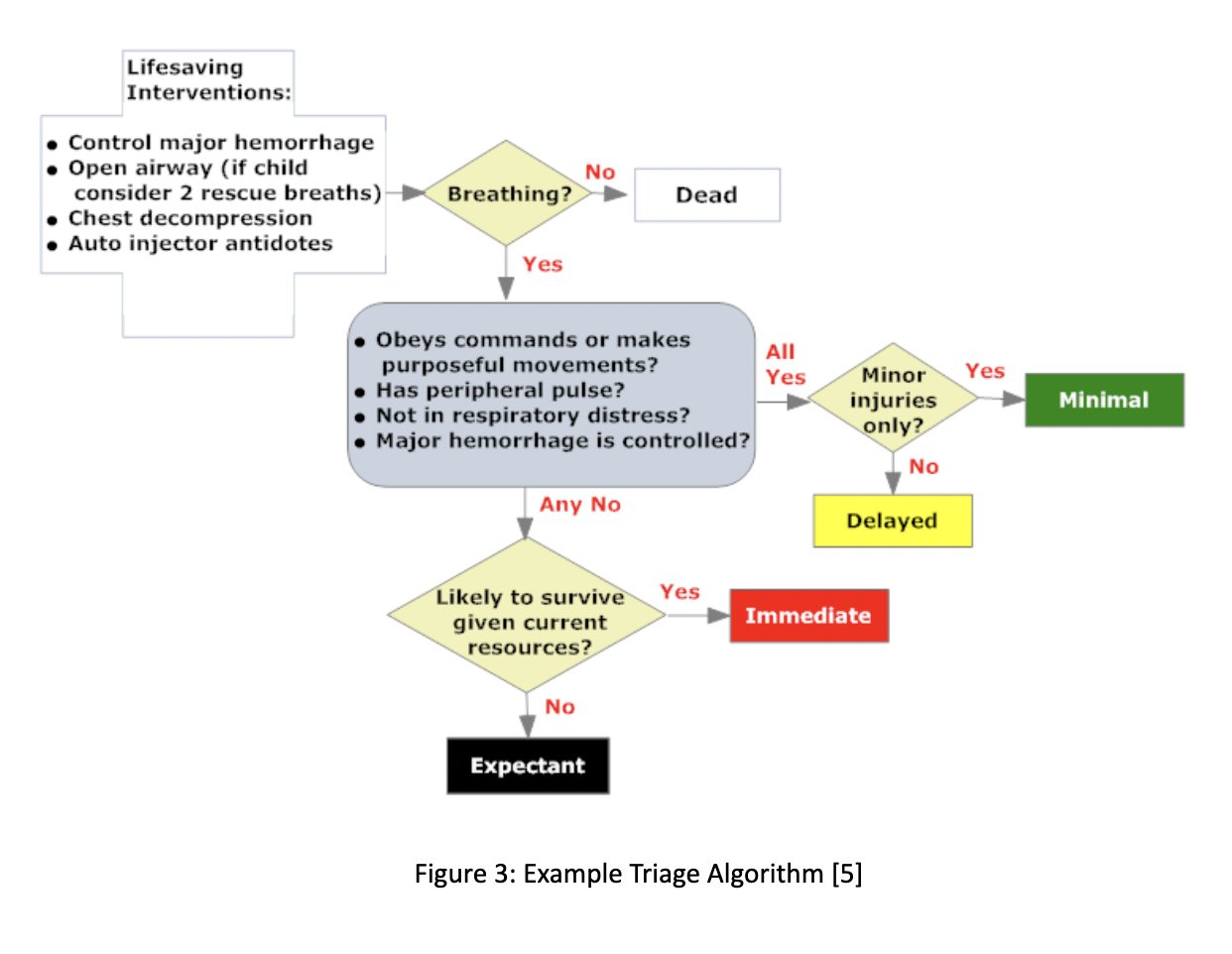









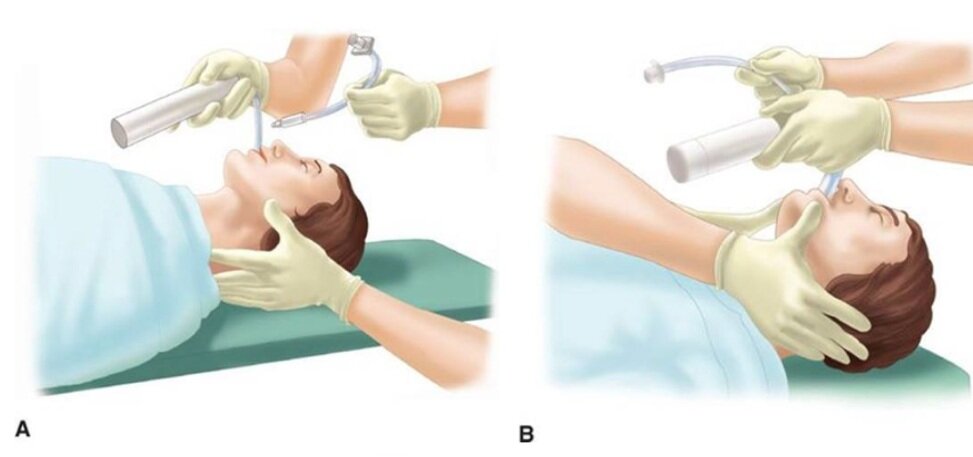






















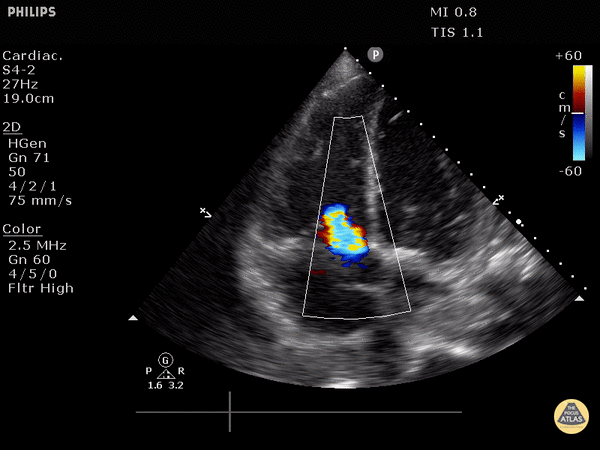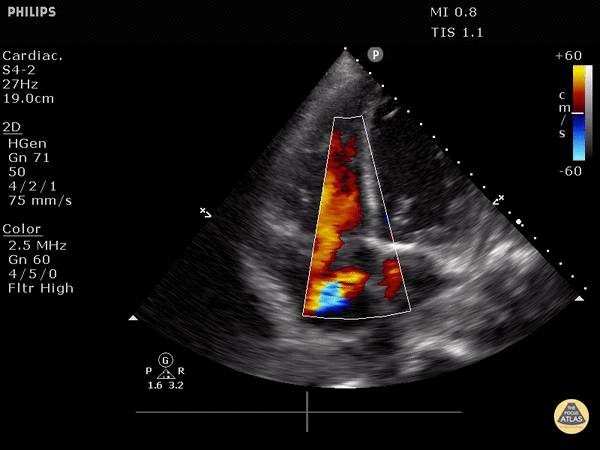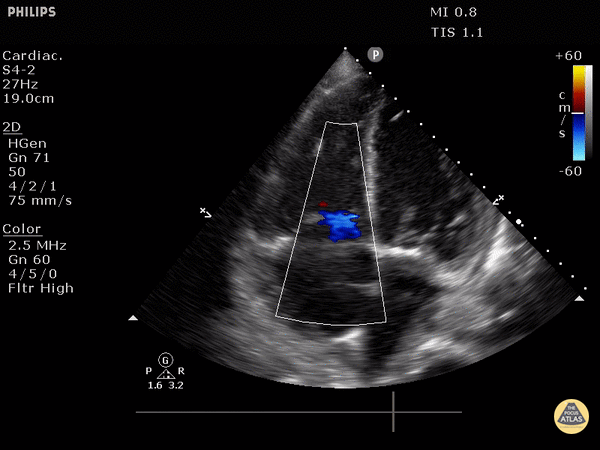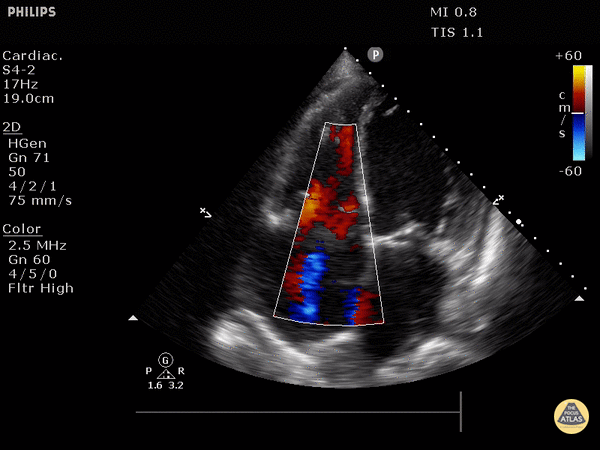




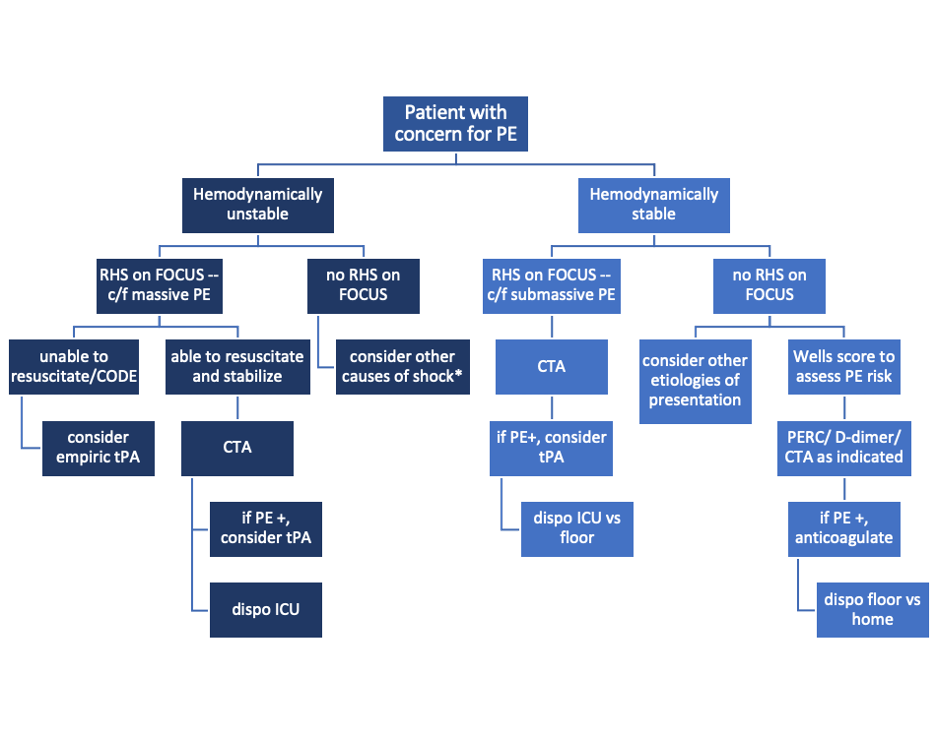









































![Figure 1: Bruising patterns that suggest child abuse. [6]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459114532-IVTA7KT3PX1J6DGC3U3L/Figure+1%3A+Patterns+of+Bruising)
![Figure 2: Forced immersion burn of buttocks with bilateral, symmetric leg involvement in a “stocking” pattern. [7]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459322548-EFHGSFQ962QP9FU5DNJN/Figure+2%3A+Burn+patterns+that+suggest+non-accidental+trauma)
![Figure 3: Classic metaphyseal lesion. White arrows denote femoral metaphyseal separation and black arrow denotes a proximal tibial lesion or “bucket handle.” [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570459580501-8461RMF0P9706GJW7XJU/Figure+3%3A+Fractures+of+NAT)
![Figure 4: Posterior and lateral rib fractures of differing ages indicative of NAT [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460464292-4QFTGMDQAB64NQBSYD9N/Figure+4%3A++Rib+fractures+of+differing+ages+indicative+of+NAT)
![Figure 5: Fundus of child with AHT with too-numerous-to-count retinal hemorrhages indicated by the black arrows. [8] The white arrow indicates small pre-retinal hemorrhages. The white arrowhead denotes hemorrhage extending into the peripheral retina…](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570460641270-3V7M52RMMV1QDMZ3PA1S/Figure+5%3A+Occular+manifestations)
![Figure 6: Elements of the Skeletal Survey. Although a full skeletal survey is currently the standard of care for patients with NAT, there are ongoing research efforts to tailor X-ray imaging more specifically to each patient. [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1570461092995-EBKSMB5DI0GTGXY0R75N/Figure+6%3A+Skeletal+surgery)