Written by: Daniel Levine, MD (NUEM ‘24) Edited by: Zach Schmitz (NUEM ‘21)
Expert Commentary by: Matt Levine, MD
The “Evidence” Behind Manual In-Line Stabilization During Intubation of Trauma Patients
Background
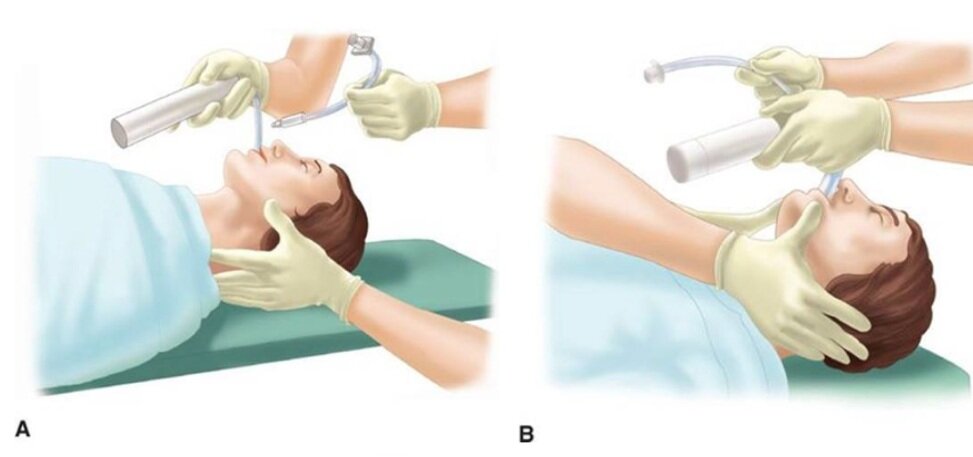
Even in the absence of frank head and neck trauma that may cause bleeding or distortions in usual anatomy, trauma patients present challenging airways because of cervical spine precautions. Standard-of-care technique according to EAST (Eastern Association for the Surgery of Trauma), West (Western Trauma Association), and ATLS (Advanced Trauma Life Support) guidelines for intubating acute trauma patients with known or potential cervical spine injury involves manual in-line stabilization (MILS). (1,2) This is a two-person technique whereby one provider performs laryngoscopy while another holds the patient’s neck in place. The two most common techniques for this procedure are depicted below, one in which the stabilizer crouches down at the head of the bed (A), and the other where the stabilizer approaches from the side of the bed (B). (3)
(photo from Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition) (3)
Evidence
Like many practices in medicine, MILS has never been studied in randomized controlled trials, and the practice stems more from weak data and expert opinion. (4) The practice of spinal stabilization began during the 1970s after a retrospective review published in 1979 of 300 patients with acute cervical injuries who presented to Johns Hopkins hospital between 1950 and 1972. Although the main focus was on the effects of laminectomy and steroids, the review also found that 11 of the 300 patients developed neurologic deficits after reaching the hospital. Of the 11 patients, 7 developed these deficits “after neck immobilization was not provided”, with no clear comment as to whether immobilization was not provided during intubation or during some other process of the patient’s care. (5) These observations led to concerns that mobilization of the neck during intubation may worsen spinal cord injury, so manual in-line stabilization became standard of care in the 1980s.
Existing data for spinal stabilization comes from trials of cadaveric models, case series, and uninjured patients. Data from cadavers with post-mortem surgically created cervical spine injuries have shown mixed results on the effects of the amount of measured movement at the injured site with versus without MILS. For example, a 1993 study by Donaldson et al. found higher degrees of subluxation and angulation at C5-C6 during orotracheal intubation without MILS compared to with stabilization in five cadaveric specimens with injuries created in that area. (6) On the other hand, a 2001 Lennarson et al. study on cadavers found MILS significantly increased subluxation in C4-C5 during the same movements. (7) While it is somewhat counterintuitive that performing MILS might be associated with increased cervical motion, this may be explained by the laryngoscopist’s need to apply greater force with the laryngoscope in order to obtain an adequate view. This is what Santoni et al. (2009) found in a matched control study of 9 patients undergoing elective surgery. The patients in this study underwent two sequential laryngoscopies and oral intubations with a Macintosh 3 blade. Pressure transducers attached to the end of the blades detected higher maximum pressures at best glottic view with MILS compared to without. (8)
What is more clear in the literature on MILS than its effect on cervical motion is that it impairs glottic visualization and subsequent first pass intubation success. In the aforementioned Donaldson study on cadavers, MILS was shown to have a negative impact on Cormack-Lehane (CL) grade. (6) Similarly, in the aforementioned Santoni et al. study of 9 patients who underwent two sequential intubations with and without MILS, glottic visualization was worse in 6 patients with MILS, and intubation failure occurred in 2 of these 6 patients compared to no intubation failures among these patients when the intubation was performed without MILS. Thiboutot et al. (2008) performed a randomized controlled trial that further demonstrated this effect. In their study, 200 elective surgical patients were randomized to receive MILS or no MILS, and the primary endpoint was rate of failed intubation at 30 seconds with a Mac 3 blade. The rate of failed intubation was half in the MILS group (50%, 47/94), significantly higher compared to the control group (5.7%, 6/105). When they released manual in-line stabilization, they were able to intubate all patients. Secondary outcomes of rate of CL grade 3-4 as well as mean latency to successful intubation were also both significantly higher in the MILS group. (9) Additionally, these data were from patients undergoing elective surgery being intubated in the controlled OR setting by anesthesiologists. It is likely that the rate of failed intubation would be even higher in the chaotic emergency department environment with an acutely injured trauma patient. While 30 seconds is a somewhat arbitrary cutoff for a failed intubation, and it is quite possible many of the patients in the MILS group who “failed” may have been successfully intubated if a longer cut-off time were chosen, hypoxia caused by failed or delayed intubation is associated with poor outcome in central nervous system injury. (10)
Conclusion
In an ideal world, a large-scale randomized controlled trial of trauma patients studying the effects of MILS on mortality and important functional neurologic outcomes would help elucidate the utility of this commonly accepted practice. However realistically, completing such a study has significant obstacles. Cervical spine injuries are relatively rare (4% of trauma injured patients)4 and only a small fraction of those cases involve unstable injuries with potentially salvageable cord function. Thus, a study with sufficient power to detect any meaningful difference in outcomes would take many thousands of patients, many trauma centers, and many years to complete. Perhaps an even larger hurdle is the ethical and medicolegal hurdle of randomizing patients to not getting MILS and possibly putting them at risk of quadriplegia. (4) So what’s a clinician to do when faced with the common scenario of having to intubate a trauma patient? I personally like the approach that Dr. Reuben Strayer discusses in his video “Advanced Airway Management for the Emergency Physician” (link below). (11) To summarize his strategy:
*The exception: in the rare situation where the patient has a highly suspected (e.g. obvious bony deformity, focal neurologic deficit) or known cervical spine injury, Dr. Strayer recommends lowering the threshold to perform a cricothyroidotomy. Additionally, he recommends considering an awake intubation approach in these patients.
Another consideration is intubating using a hyper-angulated video GlideScope, which has been shown to have improved CL views and high rates of intubation success in c-spine immobilized patients. (12) That said, occasionally equipment availability or a bloody airway may preclude the use of video laryngoscopy in the trauma setting.
References
Mayglothling J, Duane TM, Gibbs M, McCunn M, Legome E, Eastman AL, Whelan J, Shah KH; Eastern Association for the Surgery of Trauma. Emergency tracheal intubation immediately following traumatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012 Nov;73(5 Suppl 4).
Brown CVR, Inaba K, Shatz DV, Moore EE, Ciesla D, Sava JA, Alam HB, Brasel K, Vercruysse G, Sperry JL, Rizzo AG, Martin M. Western Trauma Association critical decisions in trauma: airway management in adult trauma patients. Trauma Surg Acute Care Open. 2020 Oct 9;5(1)
Leonard, J et al. "Strange and Schafermeyer's Pediatric Emergency Medicine, 4th edition." Chapter 24: Cervical Spine Injury. https://doctorlib.info/pediatric/schafermeyers-pediatric-emergency-medicine/24.html, accessed 5/7/21.
Manoach S, Paladino L. Manual in-line stabilization for acute airway management of suspected cervical spine injury: historical review and current questions. Ann Emerg Med. 2007 Sep;50(3):236-45.
Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119- 1142.
Donaldson WF 3rd, Towers JD, Doctor A, et al. A methodology to evaluate motion of the unstable spine during intubation techniques. Spine. 1993;18:2020-2023
Lennarson PJ, Smith DW, Sawin PD, Todd MM, Sato Y, Traynelis VC. Cervical spinal motion during intubation: efficacy of stabilization maneuvers in the setting of complete segmental instability. J Neurosurg. 2001 Apr;94(2 Suppl):265-70.
Santoni BG, Hindman BJ, Puttlitz CM, Weeks JB, Johnson N, Maktabi MA, Todd MM. Manual in-line stabilization increases pressures applied by the laryngoscope blade during direct laryngoscopy and orotracheal intubation. Anesthesiology. 2009 Jan;110(1):24-31.
Thiboutot, F et al. Effect of manual in-line stabilization of the C-spine on the rate of difficult orotracheal intubation by direct laryngoscopy; a randomized controlled trial. Can J Anaesth. 2009 Jun;56(6):412-8.
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993 Feb;34(2):216-22.
“Advanced Airway Management for the Emergency Physician”, uploaded by Reuben Strayer, https://vimeo.com/12440392
Bathory I, Frascarolo P, Kern C, Schoettker P. Evaluation of the GlideScope for tracheal intubation in patients with cervical spine immobilisation by a semi-rigid collar. Anaesthesia. 2009 Dec;64(12):1337-41.
Expert Commentary
So once again a review of a significant body of literature leaves a clinical question unanswered, leaving the practitioner to either follow dogma or make one’s own conclusions. Like most of our medical decision making, this is a risk/benefit analysis. So let’s go through the process.
Some background context to keep in mind:
Most cervical spine injury occurs from the initial traumatic event (primary neurologic injury). Secondary neurologic injury is a cascade of events at the cellular level that worsen primary injury and is exacerbated by hypoxia and hypercarbia, which are frequent events in difficult/prolonged intubations. These must be minimized when the brain or c spine are injured!
The movements of the cervical spine that occur during ED care pale in magnitude to the cervical spine motion that caused the primary injury to occur. These likely contribute less to neurologic outcome than secondary neurologic injury from other events during ED care like hypotension, hypoxia, and hypocarbia.
It’s too difficult to intubate with a collar on. It must be carefully and temporarily removed. As Dr. Levine taught us, MILS impairs glottic visualization and first pass intubation success. Dr. Levine also taught us that we don’t know whether the injured cervical spine actually moves less or more with MILS during intubation attempts.
The synthesis:
These factors all lead me to agree with Dr. Strayer’s approach. It is reasonable to minimize cervical spine motion as much as possible, but not at the expense of adequate glottic visualization. Maybe MILS helps minimize motion during intubation. But abandon MILS when glottic visualization is suboptimal because MILS can be contributing to this, leading to hypoxia, hypercarbia, and secondary neurologic injury. Practice MILS only until it is possibly prolonging airway success, because now it is more likely to be harming than helping.
Even more future questions remain. Much of the prior literature is based on use of traditional orotracheal intubation techniques. How much of that knowledge applies to the now widespread use of fiberoptic video intubations (i.e. Glidescope), which may have better first pass success rates and less neck motion? Do we even need to perform MILS for these intubations? Or can we reliably rapidly intubate with MILS and the Glidecope – so we can have our cake and eat it too?
Matthew Levine, MD
Associate Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern Memorial Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Levine, D. Schmitz, Z. (2021, Oct 18). C-Spine. [NUEM Blog. Expert Commentary by Levine, M]. Retrieved from http://www.nuemblog.com/blog/cervical-spine-intubation