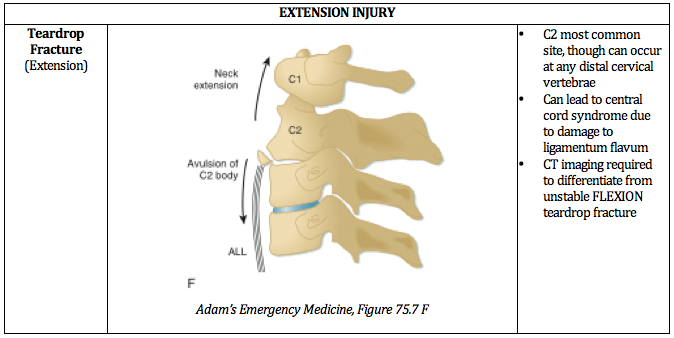
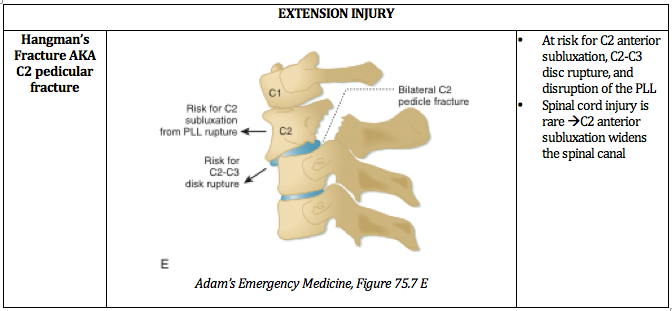
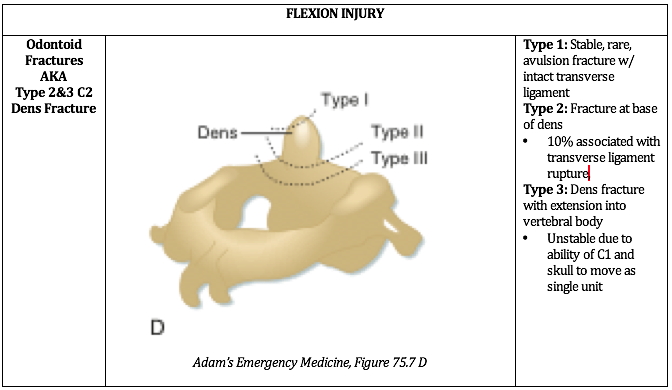
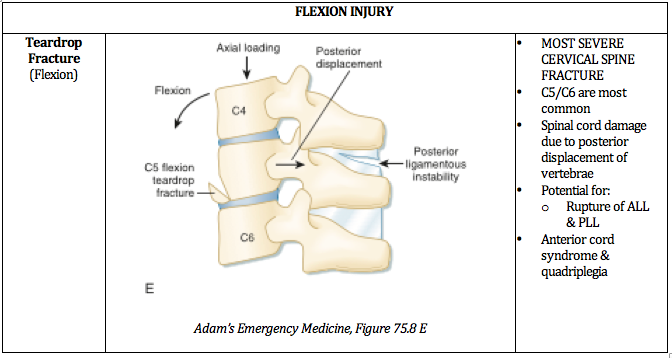
Written by: William Ford, MD, MBA (NUEM PGY-4) Edited by: Simiao Li-Sauerwine, MD (NUEM ‘18) Expert commentary by: Matthew Levine, MD
Introduction
Ankle injuries are commonly seen in emergency medicine, and serious injuries can be found in the setting of a negative X-ray. The “high ankle sprain” involves the structure of the ankle called the syndesmosis. Isolated ligamentous disruption to the syndesmosis is uncommon, though when it occurs, it is frequently missed. This can lead to serious consequences, including long-term ankle dysfunction and the need for surgery [1]. Identifying these injuries in the ED can improve recovery and facilitate prompt follow-up.
What is the Syndesmosis?
The syndesmosis is the distal articulation of the tibia and the fibula and it keeps the joint stable. For the purposes of this discussion, the focus will be on the ligamentous parts of the syndesmosis not seen on an ankle X-ray.
There are four syndesmotic ligaments: the interosseous ligament (IOL), the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), and the inferior transverse tibiofibular ligament (ITTFL) [2].
How Does Syndesmotic Injury Occur?
The motion often involved in syndesmotic injury is external rotation of the foot. Commonly, it is a combination of external rotation of the foot and excessive dorsiflexion of the ankle [3-6]. This is frequently encountered in high-speed collisions, uneven terrain, and cutting and jumping sports [1]. In most cases, syndesmotic injury will occur concurrently with a fracture, but sometimes, ligamentous injury may occur in isolation [7-8].
How Can I Diagnose Syndesmotic Injury?
Since this diagnosis is difficult to make without an MRI, providers must rely on a high index of suspicion. First, ask yourself if the mechanism of injury is consistent with syndesmosis disruption. Localizing the areas of tenderness on physical exam can also be helpful. Anterolateral or posteromedial ankle tenderness, as opposed to direct lateral or medial pain inferior to the malleoli, can suggest syndesmotic involvement. Finally, in accordance with the name “high ankle sprain”, more proximal pain may be indicative of a syndesmotic injury [1].
There are a few provocative tests described for diagnosing syndesmotic injuries. None are slam-dunk maneuvers, but a constellation of positive findings can be helpful in making the diagnosis.
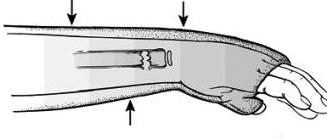
Squeeze test: With the patient sitting at the edge of the bed and knee bent at 90 degrees, a strong compressive force is applied to the tibia and fibula proximal to the mid-calf. Pain indicates a positive finding.
External rotation stress test: If the pain is reproduced with manual external rotation of the foot and ankle relative to the tibia, this test is positive. It is important to make sure the tibia is stabilized while performing this test.
Cotton test: This test is performed by attempting to translate the talus laterally under the tibia. Increased translation compared to the contralateral side or increased pain with this maneuver is a positive finding.
Fibular translation test: Stabilize the tibiotalar joint with one hand, translate the fibula anteriorly and posteriorly with the other hand. Increased pain and translation compared to the contralateral side equals a positive test.
X-ray diagnosis can also be difficult, except in the presence of frank tibiofibular diastasis [1].
Summary
Not all ankle sprains are created equal. High ankle sprains involving the syndesmosis can mean triple the recovery time of a regular sprain, chronic instability, or definitive treatment with surgery. Increased awareness and knowledge of how to diagnose these injuries is important to ensure quality care for patients.
Expert Commentary
Ankle injuries are the most common orthopedic complaint we see in the ED. While most of these presentations are simple sprains, there are other more severe injuries that will resemble uncomplicated sprains. Given the sheer volume of ankle injuries we see, we undoubtedly have all missed some of these more complicated injuries. A high ankle sprain is one of these injuries. Another classic injury that resembles an ankle sprain is the Snowboarder’s fracture. This is a fracture of the lateral process of the talus that occurs from ankle inversion and dorsiflexion. Snowboarder’s fractures clinically resemble ankle sprains and x rays miss up to 40% of these fractures! I have seen radiology miss some of these and then found the fracture by going back and magnifying the area of the lateral talus distal to the lateral malleolus on the AP and mortise views.
Regardless, plain films will not detect all Snowboarder’s fractures or high ankle sprains. It is tempting to quickly wrap and discharge all ankle “sprains” after a negative x ray. It is important, however, to discuss with these patients what is a normal healing progression and timeline. Emphasize that if 10 days go by and there is still significant pain, functional impairment, or reliance on the crutches or air cast, they need to follow up with orthopedics. Provide them with the means to arrange this follow up. It is not realistic to be able to diagnose every ankle sprain mimic in the ED, but it is our duty to provide every patient (not just ankle sprain patients) with the proper instructions for follow up in case we have missed something.
Matthew Levine, MD
Assistant Professor of Emergency Medicine
Northwestern Medicine
How To Cite This Post
[Peer-Reviewed, Web Publication] Ford W, Li-Sauerwine S. (2019, May 27). Not All Ankle Sprains are Created Equal. [NUEM Blog. Expert Commentary by Levine M]. Retrieved from http://www.nuemblog.com/blog/high-ankle-sprain.
Other Posts You May Enjoy
References
Hunt KJ, Phisitkul P, Pirolo J, et al. High Ankle Sprains and Syndesmotic Injuries in
Athletes. Journal of the American Academy of Orthopaedic Surgeons. 2015;23(11): 661-73.Fibular translation test: Stabilize the tibiotalar joint with one hand, translate the fibula anteriorly and posteriorly with the other hand. Increased pain and translation compared to the contralateral side equals a positive test.
Ogilvie-Harris DJ, Reed SC, Hedman TP. Disruption of the Ankle Syndesmosis: Biomechanical Study of the Ligamentous Restraints. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1994;10(5): 558-60.
Lin C-F, Gross MT, Weinhold P. Ankle Syndesmosis Injuries: Anatomy, Biomechanics, Mechanism of Injury, and Intervention. J. Orthop. Sport. Phys. Ther. 2006;36(6): 372–384. doi:10.2519/jospt.2006.2195.
Dattani R, Patnaik S, Kantak A, et al. Injuries to the tibiofibular syndesmosis. J. Bone Jt. Surg. 2008;90–B(4): 405–410. doi:10.1302/0301-620X.90B4.19750.
Brosky T, Nyland J, Nitz A, et al. The Ankle Ligaments: Consideration of Syndesmotic Injury and Implications for Rehabilitation. J. Orthop. Sport. Phys. Ther. 1995;21(4).
Hopkinson WJ, Pierre P St., Ryan JB, et al. Syndesmosis Sprains of the Ankle. Foot Ankle. 1990;10(6): 325–330.
Miller CD, Shelton WR, Barrett GR, et al. Deltoid and Syndesmosis Ligament of the Ankle without Fracture Injury. Am. J. Sports Med. 1995;23(6): 746–750.
Hunt KJ. Syndesmosis injuries. Curr. Rev. Musculoskelet. Med. 2013;6: 304–312. doi:10.1007/s12178-013-9184-9.