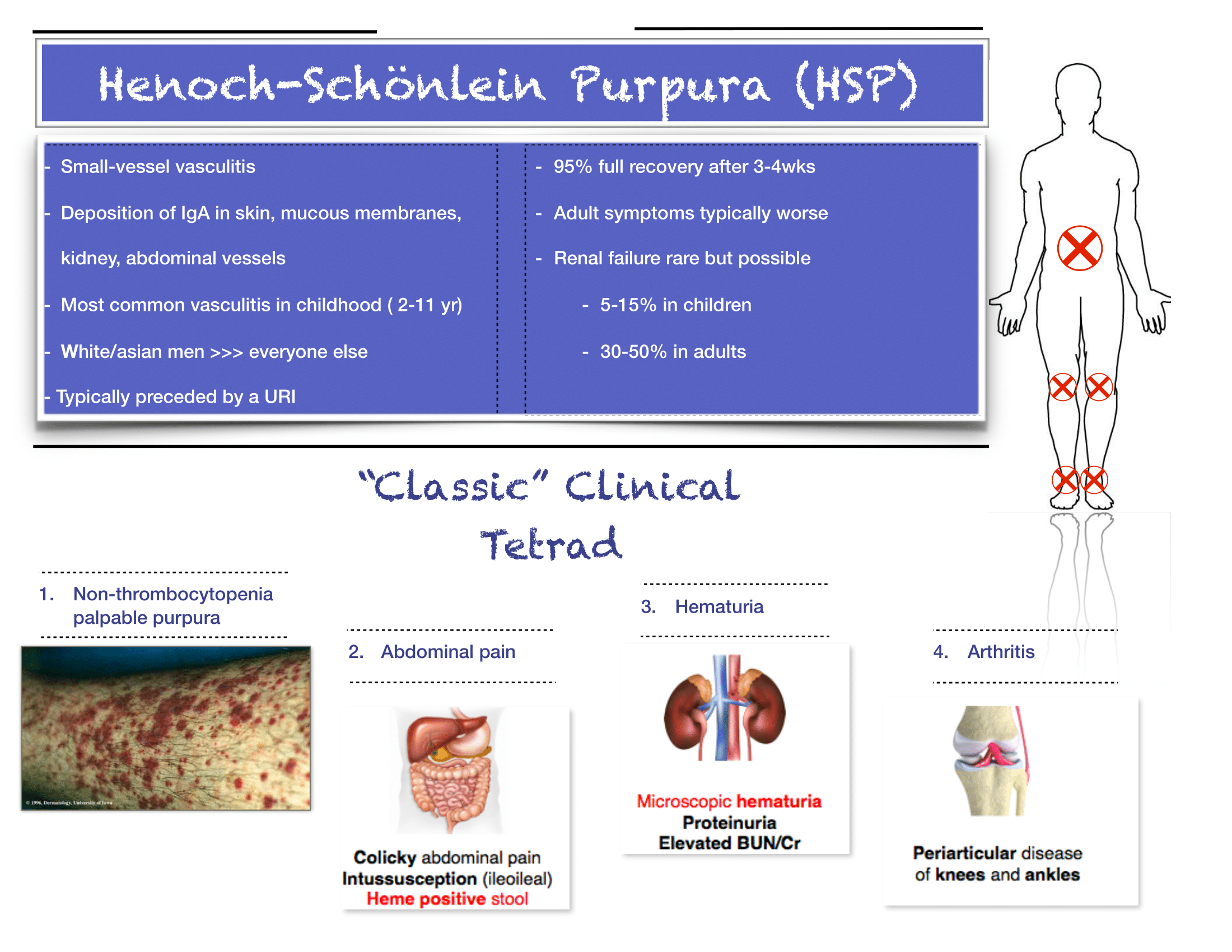
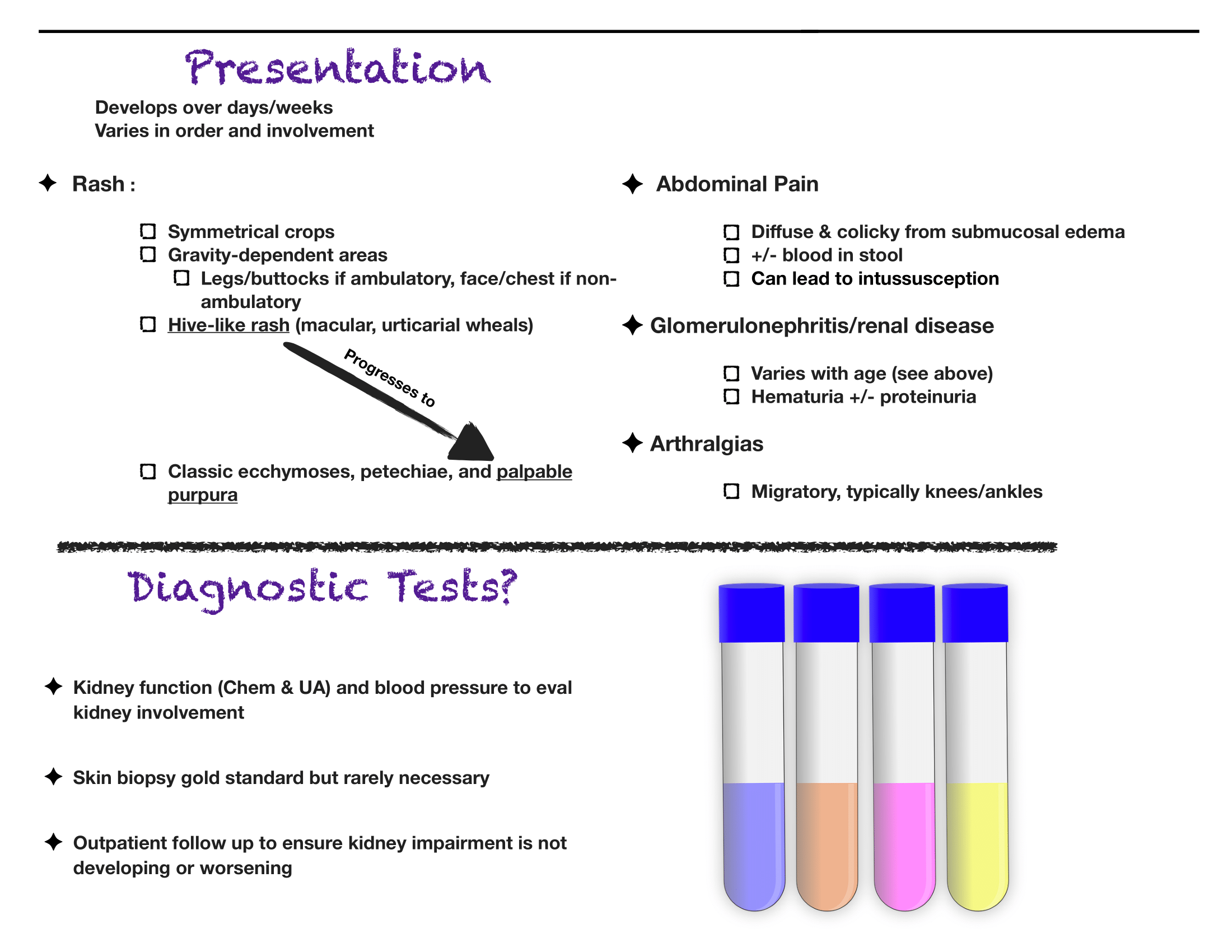
Written by: Aaron Wibberley, MD (NUEM ‘22) Edited by: Matt McCauley, MD (NUEM ‘21) Expert Commentary by: Leon Gussow, MD
Initial post
Expert Commentary
Although the large cluster of EVALI cases seen last summer and fall has subsided, the known and potential pulmonary problems associated with vaping nicotine or THC products remain an important topic for emergency practitioners and medical toxicologists alike. In a recent update on EVALI, the CDC reported that as of February 18, 2020 a total of 2807 cases had been documented from all 50 states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands. Among these cases were 68 fatalities. [1]
As this instructive post by Drs. Wibberley and McCauley suggests, many vaping liquids available at retail outlets or on the street are largely unregulated and may contain a witch’s brew of additives and contaminants whose effects on the human respiratory system have not been adequately studied. In addition to glycerin, propylene glycol, and various flavorings, inhaled vapor from these products may also contain toxic metals, formaldehyde, nitrosamines, and acrolein. [2]
One additive strongly linked to EVALI is vitamin E acetate, a synthetic oil used commercially in skin creams, dietary supplements, and multivitamins. Vitamin E acetate has been detected in many non-commercial illicit THC vaping cartridges used by EVALI patients, where it might have been added as a thickener. It was also found in bronchoalveolar lavage (BAL) fluid drawn from 48 of 51 (94%) confirmed or probable cases of EVALI, but in no such samples from 99 healthy controls. [3,4] Vitamin E acetate may impair the function of pulmonary surfactant. Despite this strong link, the CDC concluded that “evidence is not sufficient to rule out the contribution of other chemicals of concern.’ [5]
As noted in the post, since EVALI is a diagnosis of exclusion, initial clinical efforts should focus on supportive care and ruling-out other potential causes, especially pulmonary infections. Suspecting the diagnosis and establishing a connection to vaping is particularly challenging during flu season or large outbreaks of other respiratory infections. But if EVALI is not considered, a relatively stable patient with early disease may be sent home only to resume vaping. That could lead to disaster. Although new cases of EVALI have not been reported in the last several months, here’s what I think is good practice: any patient with new respiratory complaints should be asked about vaping. If they partake, they should be advised that the practice may be exacerbating their symptoms and counseled to abstain.
References
Outbreak of Lung Injury Associated with E-cigarette Use, or Vaping. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#latest-information. Accessed May 10, 2020.
Ind PW. E-cigarette or vaping product use-associated lung injury. Br J Hosp Med. 2020 Apr;81(4):1-9.
Sun LH. Contaminant found in marijuana vaping products linked to deadly lung illnesses, tests show. Washington Post Sept 6, 2019.
Blount BC et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med 2020;382:697-705.
Ghinai I et al. Characteristics of Persons Who Report Using Only Nicotine-Containing Products Among Interviewed Patients with E-cigarette, or Vaping, Product Use-Associated Lung Injury — Illinois, August-December 2019. MMWR 2020 Jan 24;69(3):84-89.
Dr. Leon Gussow, MD
Assistant Professor of Emergency Medicine, Rush University
Consultant for Illinois Poison Center
Medical Editor, The Poison Review
How To Cite This Post:
[Peer-Reviewed, Web Publication] Wibberley, A. McCauley, M. (2020, Sept 28). Vaporizing Lung Injury. [NUEM Blog. Expert Commentary by Gussow, L]. Retrieved from http://www.nuemblog.com/blog/vaporizing-lung-injury













































































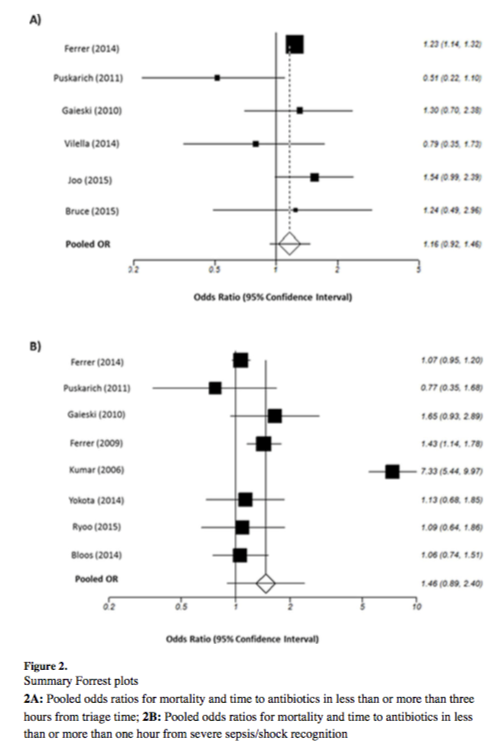
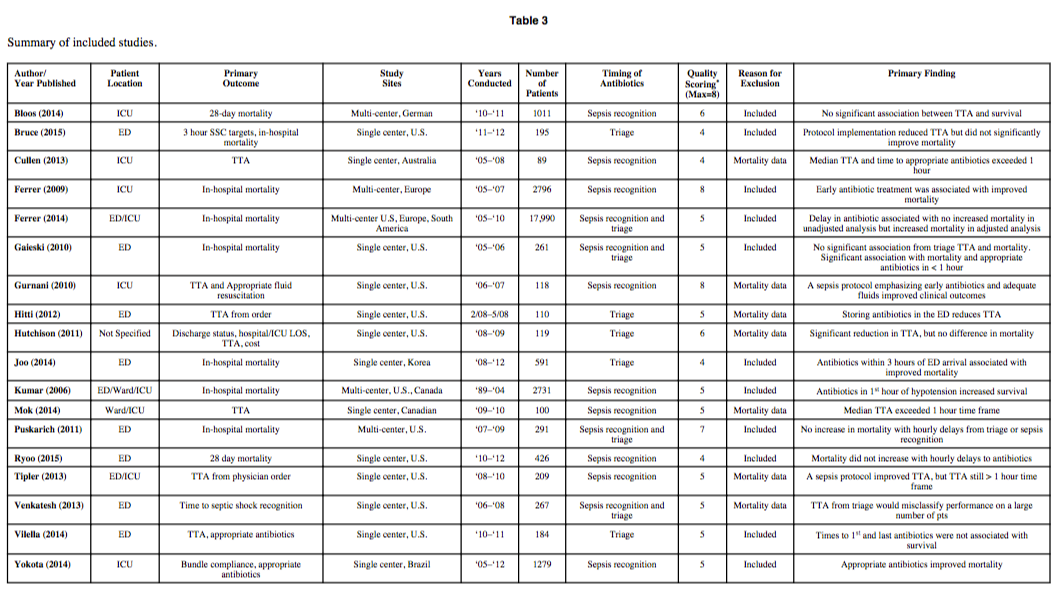





![Figure 1: Composition of Various Brands of Lipid Emulsions[1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1594923133383-9YSM79RGG2VRCXN045S4/Intralipid+image+1.png)
![Figure 2: Maximum Doses and Durations of Various Local Anesthetics[9]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1595368949352-3SYZ5X29MWCMBS3CV8BT/Screen+Shot+2020-07-21+at+5.02.19+PM.png)




![Summary of the Pathophysiology of Heat Stroke [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785361684-SVDHAE847O881FLLW493/Picture1.png)
![Subject in a cold water-immersion bath after heat- stroke [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785609566-OWN8IJYW5Y4QGFO2YPWI/Picture2.png)
![Evaporative and conductive cooling methods--note the placement of ice packs in axilla, groin as well as the cooling fan overhead [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785678671-8PWD8653TJQ61EQ1F6VS/Picture3.png)
![table of cooling methods [6]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785810930-ZUZ2S1CGXXO1ME2854ZJ/Picture4.png)
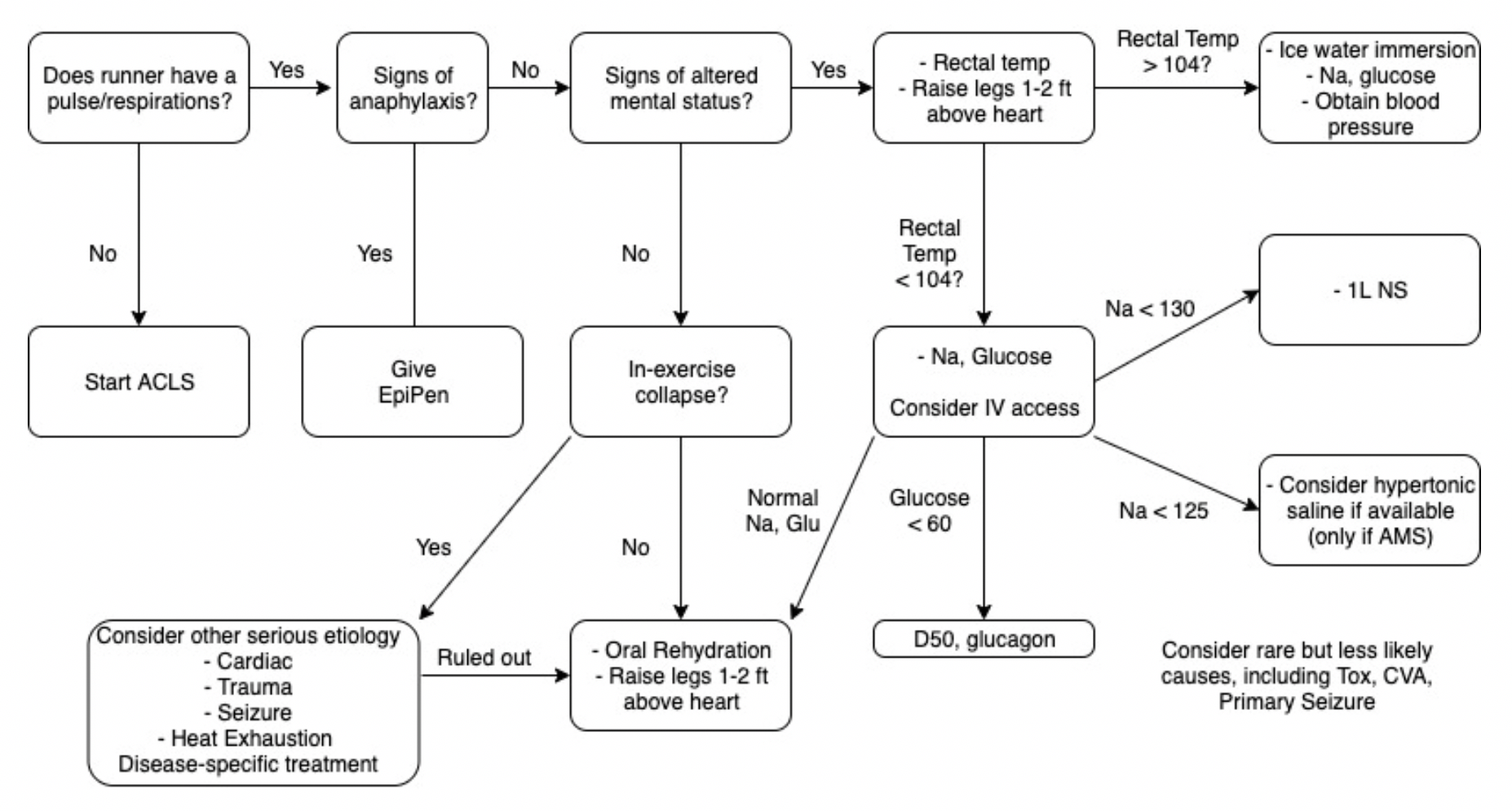
![Collapse_Algorithm[1] (1) (1).png](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593786307567-LB1EFX5KMHS371YBFKMZ/Collapse_Algorithm%5B1%5D+%281%29+%281%29.png)