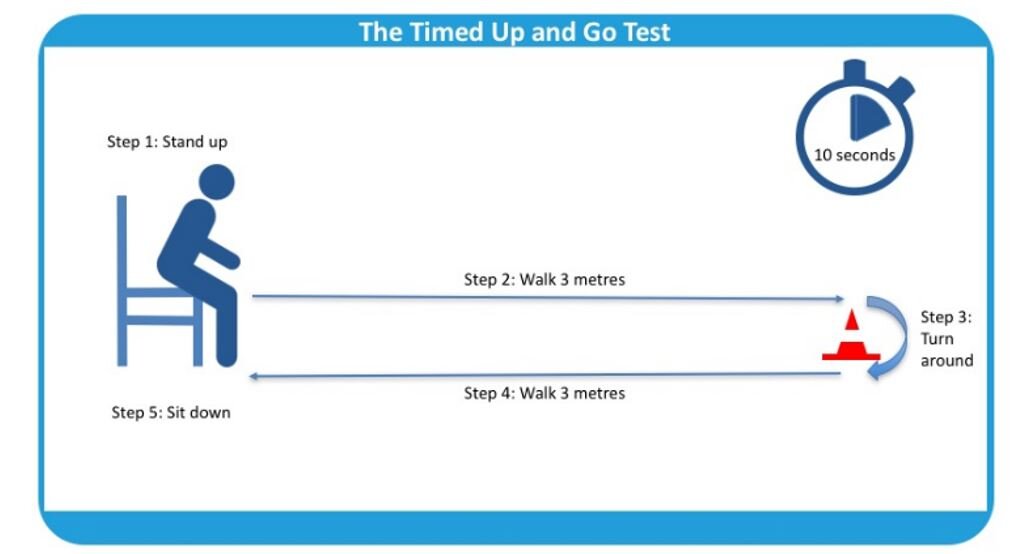
Written by: Kelsey Green, MD (NUEM ‘23) Edited by: Jordan Maivelett, MD (NUEM ‘20) Expert Commentary by: Jake Stelter, MD
Expert Commentary
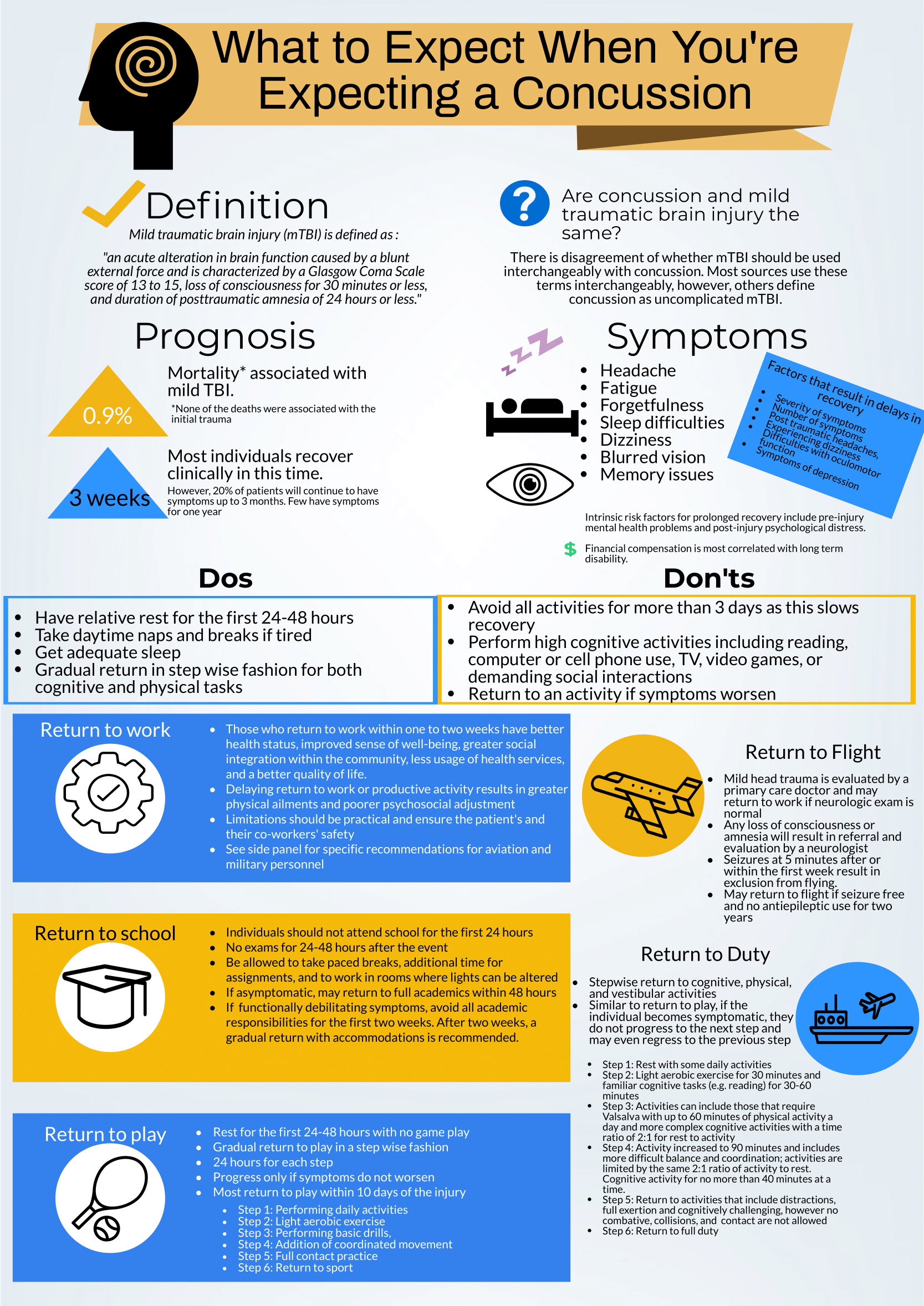
This is a great review of anticipatory guidance when counseling patients who have been diagnosed with a concussion. As noted, “mild traumatic brain injury (mTBI)” is often used synonymously with “concussion.” A better way to conceptualize this is to view concussion as a form of mTBI, realizing that mTBI can represent a spectrum of conditions. One of the most important treatments of concussion from the Emergency Department (ED) perspective is to counsel patients on what to expect and how to best control their symptoms. Concussions can present with a wide range of symptoms as detailed and can be quite distressing and disruptive to patients. As correctly pointed out, the presence of vestibular symptoms (i.e. dizziness or gait instability) as well as pre-existing mental health diagnoses, such as depression or anxiety, are associated with a protracted symptom course. Setting expectations of the symptoms they may develop and the possible timeline of symptom duration is important for patients as they manage their condition. Early conservative treatment with adequate sleep and relative cognitive and physical rest will help manage and reduce the intensity of symptoms. In our current society, it is nearly impossible to completely avoid screens and reading. Hence, “everything in moderation” is appropriate when counseling these patients. If the patient has to work at a computer, advise them to take frequent breaks for at least 10 minutes for every 30 minutes of screen time. In addition, it is recommended that patients with a concussion avoid alcohol. It is also advisable to avoid excessive caffeine. However, if a patient already uses caffeine on a daily basis, they should not stop completely, as that can lead to withdrawal headaches. Over-the-counter pain relievers, such as naproxen, ibuprofen or acetaminophen are appropriate for headache treatment, provided there are no contraindications to use.
There are multiple return-to-learn, -work and -play protocols that have been published. This is particularly applicable to athletes who have sustained a sport-related concussion (SRC). Most schools and athletic programs have protocols that have been developed in conjunction with athletic trainers and team physicians. It is important to remember that as an ED provider, you should not clear a patient to return to play. That process needs to be conducted by the school athletic trainer in collaboration with the team physician after they have had the opportunity to evaluate the patient. You should consider referring your concussion patients to a Primary Care Sports Medicine or Neurology provider for follow-up if they do not have a team physician to visit.
There are multiple free resources available to providers who are interested in learning more about concussion and educating patients. The Sport Concussion Assessment Tool – 5th Edition (SCAT5) is an in-depth evaluation tool that is often used by Sports Medicine clinicians when evaluating the extent and severity of a patient’s concussion syndrome. These resources are listed here:
References
American Medical Society for Sports Medicine position statement on concussion in sport:
https://bjsm.bmj.com/content/53/4/213
SCAT5:
https://bjsm.bmj.com/content/bjsports/early/2017/04/26/bjsports-2017-097506SCAT5.full.pdf
Jacob Stelter, MD
Emergency Medicine, Primary Care Sports Medicine
Division of Emergency Medicine
NorthShore University HealthSystem
How To Cite This Post:
[Peer-Reviewed, Web Publication] Green, K. Maivelett, J. (2021, Feb 14). What to expect when you're expecting a concussion. [NUEM Blog. Expert Commentary by Stelter, J]. Retrieved from http://www.nuemblog.com/blog/concussion.