Written by: Cameron Jones, MD (NUEM ‘23) Edited by: Vidya Eswaran, MD (NUEM ‘20) Expert Commentary by: Josh Zimmerman, MD
The Use of Imaging for Diagnosis and Management of Peritonsillar Abscesses
Among the many causes of sore throat that the EM physician may encounter, peritonsillar abscesses (PTAs) can be one of the more satisfying to diagnose and treat. A straightforward clinical diagnosis followed by a simple procedure resulting in a patient who feels much better than when they arrived...right? But what about that patient with the large, short neck and some drooling? Or the one with severe trismus giving you only the barest of glimpses at the back of their throat? Or, most feared of all, the crying child who develops lockjaw at the first glimpse of a tongue depressor? Maybe we should just get the neck CT to be on the safe side? And didn’t I hear about using ultrasound for this in some lecture?
What is a peritonsillar abscess (PTA)?
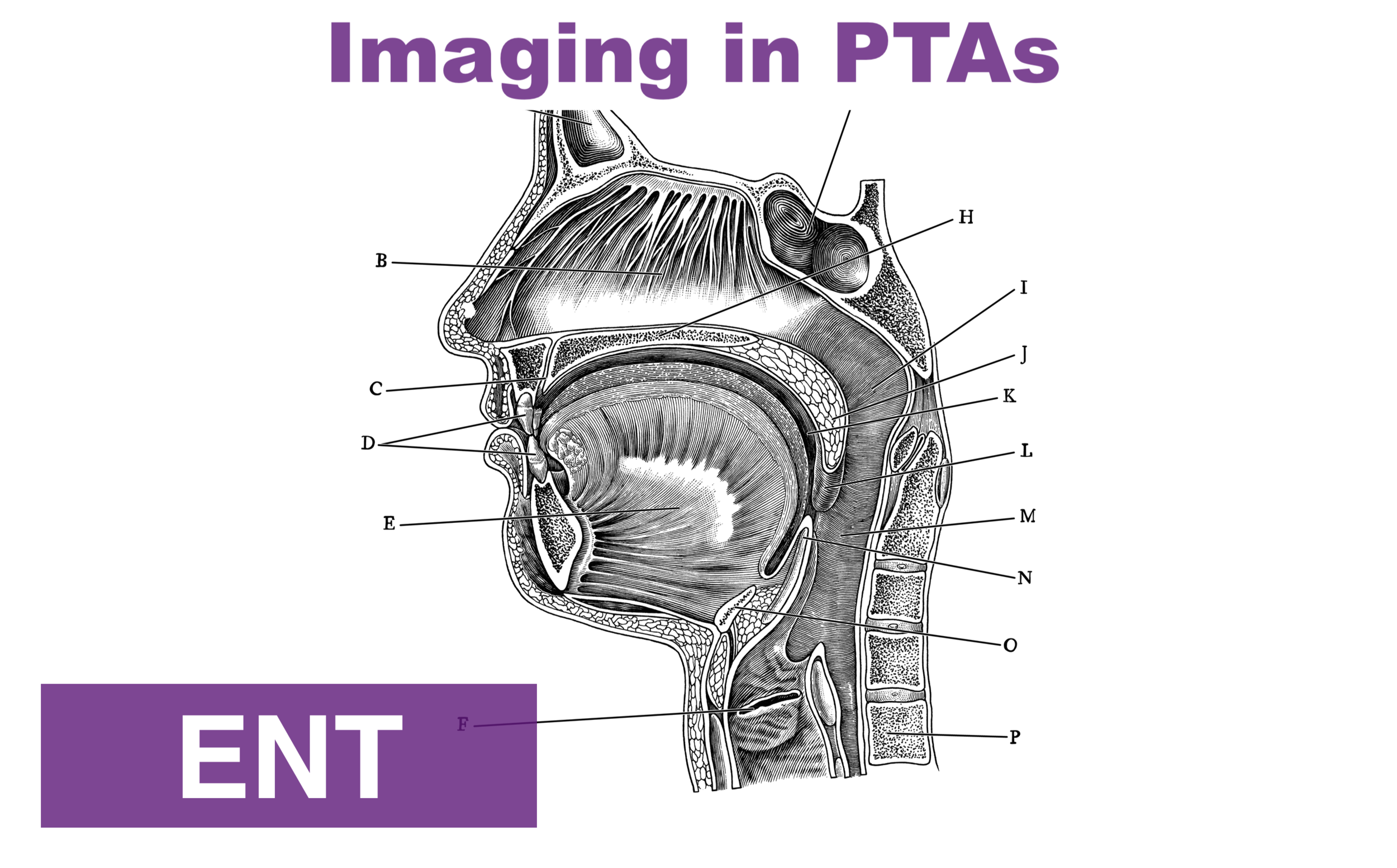
A PTA is a discrete collection of pus between the palatine tonsil capsule and the pharyngeal muscles. It should be distinguished from peritonsillar cellulitis, which is an inflammatory reaction of the same area without a definitive collection. PTAs are often preceded by tonsillitis or pharyngitis with subsequent progression of the infection. However, they may also occur due to salivary gland obstruction without preceding tonsillitis or pharyngitis. Peritonsillar abscess is often considered a clinical diagnosis based on classic symptoms and exam findings:
Throat pain (sometimes worse on the side of the abscess, but not always)
“Hot potato” or muffled voice
Unilateral swollen and erythematous tonsil +/- appreciable fluctuance
Uvula deviation
What are signs or symptoms suggestive of a more dangerous diagnosis?
Though sometimes mistakenly considered features of more concerning deep space neck infections, all of the following can also be seen with PTA:
Neck swelling
Trismus
Pooling of saliva (though this should be minor, with minimal drooling)
Other findings or symptoms of more serious deep space infections, such as retropharyngeal abscess:
Toxic appearance
Respiratory distress
Anxious appearance or leaning forward into “sniffing position”
Significant drooling
Neck pain or limited ROM out of proportion to presumed diagnosis
When should imaging be considered in the patient with suspected PTAs?
Routine imaging is not indicated for stable patients with a presumptive diagnosis based on exam. Sensitivity and specificity figures in the EM and ENT literature based on clinical exam alone are actually not very high (sensitivity <80% and specificity approximately 50%). However, these oft-cited figures are based on a comparatively small cohort of patients with presumed PTA, and in the large majority of missed diagnoses among this data, the true diagnosis is tonsillitis or peritonsillar cellulitis. CT scans, particularly contrast studies and those involving radiation of the head and neck, are not without risk, and should not be considered a screening study in well-appearing patients. Therefore, the use of imaging by ED physicians in evaluation of PTAs should really be reserved for 3 purposes:
Ruling out serious deep space neck infections, such as retropharyngeal abscesses, in a patient with signs of peritonsillar swelling but some other concerning sign or symptom, as discussed above.
- CT of the neck with contrast is best used for this purpose
Differentiating PTA from peritonsillar cellulitis or tonsillitis by identifying a discrete fluid collection
Guiding drainage in order to improve first-attempt success
- Intraoral or submandibular/transcervical ultrasounds are most appropriate for these purposes
There are few prospective studies examining the use of CT in uncomplicated PTAs, and those patients with red flags or signs of airway compromise are typically excluded. CT of the neck with IV contrast is nearly 100% sensitive and 75% specific for PTA and similarly accurate for the diagnosis of more dangerous conditions such as retropharyngeal abscesses. Increasingly, ultrasound has also become a useful option for better characterizing the location of abscesses in PTAs.
Ultrasound offers the added utility of bedside confirmation of a drainable fluid collection and, depending on provider comfort and patient tolerance, may provide real-time guidance for needle drainage. As with other applications of ultrasound, the provider must be comfortable with the technique and relevant anatomy. Prospective data indicates EM providers can become comfortable with tonsillar ultrasound technique in as few as 3-4 patients. In its use in the ED setting, ultrasound has demonstrated nearly 100% sensitivity in differentiating abscess from non-drainable inflammation or cellulitis. Thus, using ultrasound to confirm abscess in those suspected to have PTA may allow patients without drainable fluid collections to avoid unnecessary aspiration attempts.
Peritonsillar abscess seen on submandibular ultrasound. Adapted from Huang et al.
Arrows indicating edges of the abscess
T : Tonsil
* : Submandibular gland
Peritonsillar abscess on CT. Adapted from Kew et al.
Arrowheads indicating edges of the abscess.
Is imaging useful for guiding drainage of PTAs?
Ultrasound has also been studied for its utility in guiding drainage and increasing success rate of aspiration attempts. Some studies have reported low patient tolerance or mechanical challenges when using real-time intraoral ultrasound to guide drainage. However, ultrasound has also been shown to improve success-rate of aspiration attempts even when it is used for preceding visualization of the abscess and not for guided drainage.
More recently, extraoral ultrasound approaches, such as transcervical/ submandibular, have also been studied as an alternative to intraoral techniques, which can be challenging due to mechanical challenges, severe trismus, or patient discomfort. Very limited data suggests submandibular ultrasound may have lower sensitivity compared to intraoral ultrasound when evaluating PTAs, so caution is also warranted when utilizing this technique.
Intraoral ultrasound approach
(adapted from Secko, Sivitz, et al.)
Submandibular ultrasound approach
(adapted from Secko, Sivitz et al.)
What about imaging in kids?
CT scans are often ordered in pediatric patients, who may have challenging exams due to patient intolerance, and these imaging studies are particularly common in community settings where ENT expertise is not readily available. Clinical accuracy for diagnosing PTA in children appears even lower than in adults, though, as with adults, in most children incorrectly diagnosed with PTAs, the true diagnosis is tonsillitis without a drainable abscess. Many providers would also prefer to avoid the added radiation exposure of CT scans amongst this population. Thus, extraoral ultrasound approaches may be particularly helpful in pediatric patients, many of whom are unlikely to cooperate with intraoral ultrasound. Transcervical ultrasound has also been shown to reduce length of stay, CT radiation exposure, and procedures performed amongst pediatric patients with suspected PTAs, with no change in readmission rates or treatment failures. Although the extraoral ultrasound approach appears to be more technically feasible in children, use of ultrasound may also be more logistically challenging and staffing-dependent. Scans in these studies were performed and read by radiology technicians and radiologists.
So what is a reasonable approach to incorporating imaging in suspected PTAs?
The growing body of evidence described above has led to several expert recommendations that ultrasound be the first-line imaging for suspected PTAs. While there is variability in different departments regarding the ED provider’s comfort with bedside tonsillar ultrasound or, alternately, the availability of technicians and radiologists for interpreting formal ultrasounds. However, the use of ultrasound in non-toxic patients with suspected PTA has been shown to be highly effective in differentiating PTAs from peritonsillar cellulitis or tonsillitis and may save patients the discomfort and time of an unnecessary procedure. CT imaging still has its place in those patients with less certain diagnoses or concerning symptoms, but should be reserved for specific scenarios rather than being ordered routinely. The following is an evidence-based algorithm for incorporating ultrasound and CT imaging into the emergency department evaluation of these patients
* : toxic appearance, substantial drooling, respiratory distress, severe neck pain or swelling, inability to fully range neck
+ : Most patients can be safely discharged with oral antibiotics, return precautions, and ENT follow-up. Exceptions include those patients who are unable to tolerate oral medications, those with signs or symptoms of severe sepsis, patients with severe dehydration, or patient with severe comorbidities or immunocompromised state
Expert Commentary
Thank you for an excellent review of a common ED diagnosis. Sore throats are ubiquitous presenting complaints in any major ED. The final diagnosis is often uncomplicated pharyngitis, however, recognizing the early and often subtle signs of more serious conditions before a true life threat develops is a critical role for the emergency physician. While peritonsillar abscesses (PTA) in and of themselves are not typically life threatening, many of the signs and symptoms can overlap with those of more critical diagnoses such as retropharyngeal abscesses and epiglottitis.
So, that said, when should you consider imaging a patient with a suspected PTA or acute sore throat in general?
The discussion above does a thorough review evidenced based imaging practices and offers a reasonable flowsheet to guide this decision. In clinical practice imaging should help answer one of two questions:
Is a discrete fluid collection present that is amenable to drainage?
Are there findings of retropharyngeal or other deep space infection rather than a simple PTA?
I have made it my practice to consider imaging before any attempt at I&D or further care in the following circumstances:
Any patient toxic in appearance or with unstable vital signs
Any patient demonstrating signs of airway compromise
Meningismus on exam
Patients in which at PTA cannot be clearly visualized or lacking the typical secondary findings on exam
With that list in mind, let us delve into the topic a bit more in detail. Peritonsillar abscesses represent accumulation of purulent fluid which are unlikely to resolve spontaneously. Some studies have shown that drainage alone results in >90% cure rate even without antimicrobial therapy. Classically, a PTA will present with trismus, severe pharyngitis, and on pharyngeal exam a displaced tonsil, typically inferiorly and medially, as well as uvular deviation contralateral to the abscess. PTA can sometimes be confused with peritonsillar cellulitis on examination solely and is often one of the reasons clinicians opt for imaging. Peritonsillar cellulitis does not require drainage as there is no discrete fluid collection. When there is a more subtle exam, this is one scenario in which imaging may be helpful.
A practical approach that many ED physicians utilize is to consider a trial of drainage when the diagnosis is readily evident on exam. As mentioned above, when the classic findings of a displaced tonsil and uvula are present one can have a high probability of successful drainage.
Adjunct therapy – abx and steroids
The scope of this segment is meant to focus on imaging and diagnostics but it is worth a brief moment to discuss antimicrobials and adjunct therapy. While procedural drainage alone results in significant cure rates, it remains common practice to treat PTA’s with antimicrobial therapy as well. A common misconception is that PTAs are a result of Streptococcal infections. While Group A Strep is isolated from cultures, these typically tend to be polymicrobic infections with Fusobacterium additionally being a frequent culprit organism. As such, antibiotic therapy tends to be more broad spectrum with coverage of anaerobic organisms included. First line therapy remains a penicillin based antibiotic regimen. Intravenously this can be ampicillin-sulbactam (Unasyn), Piperacillin-Tazobactam (Zosyn) or Ceftriaxone Plus Metronidazole. In the penicillin allergic patient Clindamycin is a reasonable alternative. When transitioning to oral therapies, Amoxicillin-Clavulanate (Augmentin) is typically first line therapy with Clindamycin providing a reasonable alternative in penicillin allergic patients. Therapy typically is for a full 10 days.
A brief note should be made regarding steroid therapy as well. Steroids have been shown to provide significant symptomatic relief including decreasing length of symptoms and overall severity. I typically will give patients a single dose or oral or IV Dexamethasone 10 mg as part of their treatment.
Joshua Zimmerman, MD
Emergency Medicine Physician
Northwestern Lake Forest Hospital
How To Cite This Post:
[Peer-Reviewed, Web Publication] Jones, C. Eswaran, V. (2021, Jan 11). Imaging in PTAs. [NUEM Blog. Expert Commentary by Zimmerman, J]. Retrieved from http://www.nuemblog.com/blog/imaging-in-PTAs.
Other Posts You May Enjoy
References
Carratola, M. C., Frisenda, G., Gastanaduy, M., & Lindhe Guarisco, J. (2019). Association of Computed Tomography With Treatment and Timing of Care in Adult Patients With Peritonsillar Abscess. Ochsner Journal, 19, 309–313. https://doi.org/10.31486/toj.18.0168
Costantino, T. G., Satz, W. A., Dehnkamp, W., & Goett, H. (2012). Randomized Trial Comparing Intraoral Ultrasound to Landmark-based Needle Aspiration in Patients with Suspected Peritonsillar Abscess. Academic Emergency Medicine, 19(6), 626–631. https://doi.org/10.1111/j.1553-2712.2012.01380.x
Cunha, B., Filho, A., Sakae, F. A., Sennes, L. U., Imamura, R., & De Menezes, M. R. (n.d.). Intraoral and transcutaneous cervical ultrasound in the differential diagnosis of peritonsillar cellulitis and abscesses Summary. Brazilian Journal of Otorhinolaryngology, 72(3), 377-81. http://www.rborl.org.br/
Fordham, M. T., Rock, A. N., Bandarkar, A., Preciado, D., Levy, M., Cohen, J., … Reilly, B. K. (2015). Transcervical ultrasonography in the diagnosis of pediatric peritonsillar abscess. The Laryngoscope, 125(12), 2799–2804. https://doi-org/10.1002/lary.25354
Froehlich, M. H., Huang, Z., & Reilly, B. K. (2017, April 1). Utilization of ultrasound for diagnostic evaluation and management of peritonsillar abscesses. Current Opinion in Otolaryngology and Head and Neck Surgery. Lippincott Williams and Wilkins. https://doi.org/10.1097/MOO.0000000000000338
Herzon, F. S., & Martin, A. D. (2006). Medical and Surgical Treatment of Peritonsillar, Retropharyngeal, and Parapharyngeal Abscesses. Current Infectious Disease Reports, 8:196–202. https://doi.org/10.1007/s11908-006-0059-8
Huang, Z., Vintzileos, W., Gordish-Dressman, H., Bandarkar, A., & Reilly, B. K. (2017). Pediatric peritonsillar abscess: Outcomes and cost savings from using transcervical ultrasound. The Laryngoscope, 127(8), 1924–1929. https://doi.org/10.1002/lary.26470
J Scott, P. M., Loftus, W. K., Kew, J., Ahum, A., Yue, V., & Van Hasselt, C. A. (2020). Diagnosis of peritonsillar infections: a prospective study of ultrasound, computerized tomography and clinical diagnosis. The Journal of Laryngology and Otology, 113, 229–232. https://doi.org/10.1017/S0022215100143634
Kew, J., Ahuja, A., Loftus, W. K., Scott, P. M. J., & Metreweli, C. (1998). Peritonsillar Abscess Appearance on Intra-oral Ultrasonography. Clinical Radiology (Vol. 53).
Lyon, M., & Blaivas, M. (2005). Intraoral Ultrasound in the Diagnosis and Treatment of Suspected Peritonsillar Abscess in the Emergency Department. Academic Emergency Medicine, 12(1), 85–88. https://doi.org/10.1111/j.1553-2712.2005.tb01485.x
Nogan, S., Jandali, D., Cipolla, M., & DeSilva, B. (2015). The use of ultrasound imaging in evaluation of peritonsillar infections. The Laryngoscope, 125(11), 2604–2607. https://doi.org/10.1002/lary.25313
Patel, K. S., Ahmad, S., O’leary, G., & Michel, M. (1992). The role of computed tomography in the management of peritonsillar abscess. Otolaryngology--Head and Neck Surgery, 107(6), 727-732. https://doi.org/10.1177/019459988910700603.1
Powell, J., & Wilson, J. A. (2012). An evidence-based review of peritonsillar abscess. Clinical Otolaryngology, 37(2), 136–145. http://doi.wiley.com/10.1111/j.1749-4486.2012.02452.x
Salihoglu, M., Eroglu, M., Osman Yildirim, A., Cakmak, A., Hardal, U., & Kara, K. (2013). Transoral ultrasonography in the diagnosis and treatment of peritonsillar abscess. https://doi.org/10.1016/j.clinimag.2012.09.023
Valdez, T. and Vallejo, J., 2016. Infectious Diseases In Pediatric Otolaryngology. Springer International Publishing.